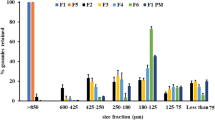
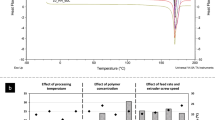
Abstract
The development of oral solid dosage forms, such as tablets that contain a high dose of drug(s), requires polymers and other additives to be incorporated at low levels as possible, to keep the final tablet weight low, and, correspondingly, the dosage form size small enough to be acceptable from a patient perspective. Additionally, a multi-step batch-based manufacturing process is usually required for production of solid dosage forms. This study presents the development and production, by twin-screw melt granulation technology, of a high-dose immediate-release fixed-dose combination (FDC) product of metformin hydrochloride (MET) and sitagliptin phosphate (SIT), with drug loads of 80% w/w and 6% w/w, respectively. For an 850/63 mg dose of MET/SIT, the final weight of the caplets was approximately 1063 mg compared with 1143 mg for the equivalent dose in Janumet®, the marketed product. Mixtures of the two drugs and polymers were melt-granulated at temperatures below the individual melting temperatures of MET and SIT (231.65 and 213.89°C, respectively) but above the glass transition temperature or melting temperature of the binder(s) used. By careful selection of binders, and processing conditions, direct compressed immediate-release caplets with desired product profiles were successfully produced. The melt granule formulations before compression showed good flow properties, were larger in particle size than individual starting API materials and were easily compressible. Melt granulation is a suitable platform for developing direct compressible high-dose immediate-release solid dosage forms of FDC products.






Similar content being viewed by others
References
American Diabetes Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care [Internet]. 2011 Jan 1 [cited 2018 Mar 15];34 Suppl 1(Supplement 1):S62–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21193628.
Mayo Clinic. Diabetes—symptoms and causes - Mayo Clinic [Internet]. 2018 [cited 2018 Mar 21]. Available from: https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444.
Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature [Internet]. 2001 [cited 2018 Mar 22];414:782–7. Available from: https://www.nature.com/articles/414782a.pdf.
Merck. Glucophage film-coated tablets—summary of product characteristics (SPC) [Internet]. 2016 [cited 2018 Mar 26]. Available from: http://www.medicines.ie/medicine/3578/SPC/Glucophage+Film-Coated+Tablets/#PHARMACODYNAMIC_PROPS.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet [Internet]. 2006 Nov 11 [cited 2018 Mar 26];368(9548):1696–705. Available from: https://www.sciencedirect.com/science/article/pii/S0140673606697055.
Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65(5):795–6.
Cheong C, Barner JC, Lawson KA, Johnsrud MT. Patient adherence and reimbursement amount for antidiabetic fixed-dose combination products compared with dual therapy among texas medicaid recipients. Clin Ther [Internet]. 2008 Oct 1 [cited 2019 Jan 17];30(10):1893–907. Available from: https://www.sciencedirect.com/science/article/pii/S014929180800324X.
Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med [Internet]. 2008 [cited 2019 Jan 17];23(5):611–4. Available from: https://link.springer.com/article/10.1007/s11606-008-0544-x
Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agents. Am J Health Syst Pharm [Internet]. 2007 Jun 15 [cited 2019 Jan 17];64(12):1279–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17563050.
Shanmugam S. Granulation techniques and technologies: recent progresses. Bioimpacts [Internet]. 2015 [cited 2019 Mar 25];5(1):55–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25901297
Schaefer T, Holm P, Kristensen HG. Melt granulation in a laboratory scale high shear mixer. Drug Dev Ind Pharm [Internet]. 1990 Jan 20 [cited 2019 Sep 23];16(8):1249–77. Available from: http://www.tandfonline.com/doi/full/10.3109/03639049009115960.
Evrard B, Amighi K, Beten D, Delattre L, Moës AJ. Influence of melting and rheological properties of fatty binders on the melt granulation process in a high-shear mixer. Drug Dev Ind Pharm [Internet]. 1999 Jan 22 [cited 2019 Sep 23];25(11):1177–84. Available from: http://www.tandfonline.com/doi/full/10.1081/DDC-100102285.
Vasanthavada M, Wang Y, Haefele T, Lakshman JP, Mone M, Tong W, et al. Application of melt granulation technology using twin-screw extruder in development of high-dose modified-release tablet formulation. J Pharm Sci [Internet]. 2011 May 1 [cited 2019 Mar 25];100(5):1923–34. Available from: https://www.sciencedirect.com/science/article/pii/S0022354915321626.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33(10):1043–57.
Vaingankar P, Amin P. Continuous melt granulation to develop high drug loaded sustained release tablet of Metformin HCl. Asian J Pharm Sci [Internet]. 2017 Jan 1 [cited 2019 Mar 25];12(1):37–50. Available from: https://www.sciencedirect.com/science/article/pii/S1818087616300769.
Batra A, Desai D, Serajuddin ATM. Investigating the use of polymeric binders in twin screw melt granulation process for improving compactibility of drugs. J Pharm Sci [Internet]. 2017 Jan 1 [cited 2019 May 2];106(1):140–50. Available from: https://www.sciencedirect.com/science/article/pii/S0022354916416011.
Lakshman JP, Kowalski J, Vasanthavada M, Tong W-Q, Joshi YM, Serajuddin ATM. Application of melt granulation technology to enhance tabletting properties of poorly compactible high-dose drugs. J Pharm Sci [Internet]. 2011 Apr 1 [cited 2019 Mar 25];100(4):1553–65. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354915322024.
Arndt OR, Kleinebudde P. Influence of binder properties on dry granules and tablets. Powder Technol [Internet]. 2018 Sep 1 [cited 2019 Mar 28];337:68–77. Available from: https://www.sciencedirect.com/science/article/pii/S0032591017303522.
Mettler Toledo. Evaluation possibilities for the glass transition—METTLER TOLEDO [Internet]. 2019 [cited 2019 Sep 30]. Available from: https://www.mt.com/hk/en/home/supportive_content/matchar_apps/MatChar_HB401.html.
Freeman R. Measuring the flow properties of consolidated, conditioned and aerated powders—a comparative study using a powder rheometer and a rotational shear cell. Powder Technol [Internet]. 2007 May 16 [cited 2019 Apr 8];174(1–2):25–33. Available from: https://www.sciencedirect.com/science/article/pii/S0032591006004475.
Pitt KG, Heasley MG. Determination of the tensile strength of elongated tablets. Powder Technol [Internet]. 2013 Apr 1 [cited 2019 Apr 16];238:169–75. Available from: https://www.sciencedirect.com/science/article/pii/S0032591011007467.
British Pharmacopoeia. Appendix XVII G. Friability—British Pharmacopoeia [Internet]. 2019 [cited 2019 Apr 17]. Available from: https://www.pharmacopoeia.com/bp-2019/appendices/appendix-17/appendix-xvii-g%2D%2Dfriability.html?date=2019-04-01&text=friability.
British Pharmacopoeia. Appendix XII A. Disintegration—British Pharmacopoeia [Internet]. 2019 [cited 2019 Apr 17]. Available from: https://www.pharmacopoeia.com/bp-2019/appendices/appendix-12/appendix-xii-a%2D%2Ddisintegration.html?date=2019-04-01#f20901.
Sarkar D, Nandi G, Changder A, Hudati P, Sarkar S, Ghosh LK. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int J Biol Macromol [Internet]. 2017 Mar [cited 2019 Mar 28];96:137–48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27956100.
Priyadarshini R, Nandi G, Changder A, Chowdhury S, Chakraborty S, Ghosh LK. Gastroretentive extended release of metformin from methacrylamide-g-gellan and tamarind seed gum composite matrix. Carbohydr Polym [Internet]. 2016 Feb 10 [cited 2019 Mar 28];137:100–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26686110.
Sarode A, Wang P, Cote C, Worthen DR. Low-viscosity hydroxypropylcellulose (HPC) grades SL and SSL: versatile pharmaceutical polymers for dissolution enhancement, controlled release, and pharmaceutical processing. AAPS PharmSciTech [Internet]. 2013 Mar [cited 2019 Apr 18];14(1):151. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23250708.
Picker-Freyer KM, Dürig T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS PharmSciTech [Internet]. 2007 Oct 9 [cited 2019 Apr 1];8(4):82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18181552.
Rebitski EP, Aranda P, Darder M, Carraro R, Ruiz-Hitzky E. Intercalation of metformin into montmorillonite. Dalt Trans [Internet]. 2018 Feb 27 [cited 2019 Apr 2];47(9):3185–92. Available from: http://xlink.rsc.org/?DOI=C7DT04197G.
Ige PP, Gattani SG. Design and in vitro and in vivo characterization of mucoadhesive matrix pellets of metformin hydrochloride for oral controlled release: a technical note. Arch Pharm Res [Internet]. 2012 Mar 5 [cited 2019 Apr 2];35(3):487–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22477196.
Shantikumar S, Sreekanth G, SurendraNath K V., JaferValli S, Satheeshkumar N. Compatibility study between sitagliptin and pharmaceutical excipients used in solid dosage forms. J Therm Anal Calorim [Internet]. 2014 Mar 30 [cited 2019 Apr 2];115(3):2423–8. Available from: http://link.springer.com/10.1007/s10973-013-3329-3
Baird JA, Olayo-Valles R, Rinaldi C, Taylor LS. Effect of molecular weight, temperature, and additives on the moisture sorption properties of polyethylene glycol. J Pharm Sci [Internet]. 2010 Jan 1 [cited 2019 Apr 2];99(1):154–68. Available from: https://www.sciencedirect.com/science/article/pii/S0022354916303719?via%3Dihub.
Kelleher JF, Gilvary GC, Madi AM, Jones DS, Li S, Tian Y, et al. A comparative study between hot-melt extrusion and spray-drying for the manufacture of anti-hypertension compatible monolithic fixed-dose combination products. Int J Pharm [Internet]. 2018 Jul 10 [cited 2019 May 2];545(1–2):183–96. Available from: https://www.sciencedirect.com/science/article/pii/S0378517318303016.
Parikh DM. Handbook of pharmaceutical granulation technology [Internet]. Informa Healthcare USA; 2010 [cited 2019 Apr 2]. 659 p. Available from: https://www.crcpress.com/Handbook-of-Pharmaceutical-Granulation-Technology/Parikh/p/book/9781439807897.
Passerini N, Albertini B, González-Rodríguez ML, Cavallari C, Rodriguez L. Preparation and characterisation of ibuprofen-poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci [Internet]. 2002 Feb [cited 2019 Apr 2];15(1):71–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11803133.
Shimpi S, Chauhan B, Mahadik KR, Paradkar A. Preparation and evaluation of diltiazem hydrochloride-Gelucire 43/01 floating granules prepared by melt granulation. AAPS PharmSciTech [Internet]. 2004 Jul 12 [cited 2019 Apr 2];5(3):e43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15760076.
Schæfer T, Mathiesen C. Melt pelletization in a high shear mixer. VIII. Effects of binder viscosity. Int J Pharm [Internet]. 1996 Aug 9 [cited 2019 Apr 3];139(1–2):125–38. Available from: https://www.sciencedirect.com/science/article/pii/0378517396045498.
Van Melkebeke B, Vermeulen B, Vervaet C, Remon JP. Melt granulation using a twin-screw extruder: a case study. Int J Pharm [Internet]. 2006 Dec 1 [cited 2019 Apr 3];326(1–2):89–93. Available from: https://www.sciencedirect.com/science/article/pii/S0378517306005576.
Wagner JR, Mount EM, Giles HF, Wagner JR, Mount EM, Giles HF. Troubleshooting mechanical extrusion problems. Extrusion [Internet]. 2014 Jan 1 [cited 2019 May 20];315–27. Available from: https://www.sciencedirect.com/science/article/pii/B9781437734812000272.
Childs SL, Chyall LJ, Bretnall AE, Clarke SG, Dunlap JT, Coates DA, et al. A metastable polymorph of metformin hydrochloride: isolation and characterization using capillary crystallization and thermal microscopy techniques. Cryst Growth Des [Internet]. 2004 [cited 2019 Apr 17];4(3):441–9. Available from: https://pubs.acs.org/doi/abs/10.1021/cg034243p.
Bretnall AE, Clarke SG. In: Brittain HG, editor. Metformin hydrochloride—analytical profiles of drug substances and excipients, vol. 25: Academic Press; 1998. p. 243–93.
Garekani HA, Ford JL, Rubinstein MH, Rajabi-Siahboomi AR. Formation and compression characteristics of prismatic polyhedral and thin plate-like crystals of paracetamol. Int J Pharm [Internet]. 1999 Sep 30 [cited 2019 Apr 17];187(1):77–89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10502615.
Lindberg N, Pålsson M, Pihl A, Freeman R, Freeman T, Zetzener H, et al. Flowability measurements of pharmaceutical powder mixtures with poor flow using five different techniques. Drug Dev Ind Pharm [Internet]. 2004 Jan 9 [cited 2019 Apr 8];30(7):785–91. Available from: http://www.tandfonline.com/doi/full/10.1081/DDC-120040343.
Freeman RE, Cooke JR, Schneider LCR. Measuring shear properties and normal stresses generated within a rotational shear cell for consolidated and non-consolidated powders. Powder Technol [Internet]. 2009 Mar 5 [cited 2019 Apr 8];190(1–2):65–9. Available from: https://www.sciencedirect.com/science/article/pii/S0032591008002532.
Guo Z, Chen X, Xu Y, Liu H. Effect of granular shape on angle of internal friction of binary granular system. Fuel [Internet]. 2015 Jun 15 [cited 2019 Apr 8];150:298–304. Available from: https://www.sciencedirect.com/science/article/pii/S0016236115001957.
Podczeck F, Mia Y. The influence of particle size and shape on the angle of internal friction and the flow factor of unlubricated and lubricated powders. Int J Pharm [Internet]. 1996 Nov 29 [cited 2019 Apr 11];144(2):187–94. Available from: https://www.sciencedirect.com/science/article/pii/S0378517396047552.
Wang Y, Koynov S, Glasser BJ, Muzzio FJ. A method to analyze shear cell data of powders measured under different initial consolidation stresses. Powder Technol [Internet]. 2016 Jun [cited 2019 Apr 8];294(294):105–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0032591016300687.
Leung LY, Mao C, Srivastava I, Du P, Yang C-Y. Flow function of pharmaceutical powders is predominantly governed by cohesion, not by friction coefficients. J Pharm Sci [Internet]. 2017 Jul [cited 2019 Apr 8];106(7):1865–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354917302447.
Signet. Avicel PH [Internet]. 2019 [cited 2019 May 20]. Available from: http://www.signetchem.com/product.aspx?prdid=2.
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—a review. Int J Pharm [Internet]. 2014 Oct 1 [cited 2019 May 20];473(1–2):64–72. Available from: https://www.sciencedirect.com/science/article/pii/S0378517314004840.
Jenike AW. Storage and flow of solids. Bull 123, Eng Exp Station Univ Utah, USA. 1964.
British Pharmacopoeia. Metformin tablets—British Pharmacopoeia [Internet]. 2019 [cited 2019 Apr 16]. Available from: https://www.pharmacopoeia.com/bp-2019/formulated-specific/metformin-tablets.html?date=2019-01-01&text=Metformin.
British Pharmacopoeia. Sitagliptin tablets—British Pharmacopoeia [Internet]. 2019 [cited 2019 Apr 16]. Available from: https://www.pharmacopoeia.com/bp-2019/formulated-specific/sitagliptin-tablets.html?date=2019-01-01&text=sitagliptin.
Fell JT, Newton JM. Determination of tablet strength by the diametral-compression test. J Pharm Sci [Internet]. 1970 May 1 [cited 2019 Apr 17];59(5):688–91. Available from: https://www.sciencedirect.com/science/article/pii/S0022354915373068.
Seitz JA, Flessland GM. Evaluation of the physical properties of compressed tablets. I: Tablet hardness and friability. J Pharm Sci [Internet]. 1965 Sep 1 [cited 2019 Apr 17];54(9):1353–7. Available from: https://www.sciencedirect.com/science/article/pii/S0022354915350899.
Chowhan ZT. Moisture, hardness, disintegration and dissolution interrelationships in compressed tablets prepared by the wet granulation process. Drug Dev Ind Pharm [Internet]. 1979 Jan 20 [cited 2019 Apr 17];5(1):41–62. Available from: http://www.tandfonline.com/doi/full/10.3109/03639047909055661.
Markl D, Zeitler JA. A Review of disintegration mechanisms and measurement techniques. Pharm Res [Internet]. 2017 [cited 2019 Apr 18];34(5):890–917. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28251425.
Caramella C, Colombo P, Conte U, Ferrari F, Gazzaniga A, LaManna A, et al. A physical analysis of the phenomenon of tablet disintegration. Int J Pharm [Internet]. 1988 Jun 1 [cited 2019 Apr 17];44(1–3):177–86. Available from: https://www.sciencedirect.com/science/article/pii/0378517388901147.
Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol [Internet]. 2015 May 15 [cited 2019 Apr 17];1–12. Available from: http://www.tandfonline.com/doi/full/10.3109/10837450.2015.1045618.
Radwan A, Wagner M, Amidon G, Langguth P. Bio-predictive tablet disintegration: effect of water diffusivity, fluid flow, food composition and test conditions. Eur J Pharm Sci [Internet]. 2014 [cited 2019 Apr 17];57:273–9. Available from: http://europepmc.org/abstract/med/24036239.
British Pharmacopoeia. Tablets—British Pharmacopoeia [Internet]. 2019 [cited 2019 Apr 18]. Available from: https://www.pharmacopoeia.com/Publication/bp-2019/formulated-general/tablets.html?date=2019-04-01&text=disintegration.
European Medicines Agency. M9 Step 2b on biopharmaceutics classification system based biowaivers [Internet]. 2019 [cited 2019 Jan 10]. Available from: www.ema.europa.eu/contact.
Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res [Internet]. 2005 Jan 1 [cited 2019 Jan 11];22(1):11–23. Available from: http://link.springer.com/10.1007/s11095-004-9004-4.
Acknowledgements
This investigation was conducted with the financial support of both the Department for the Economy of Northern Ireland and Science Foundation of Ireland (SFI) under Grant Number 14/IA/2559.
AM Healy also acknowledges funding from Science Foundation Ireland (SFI) [grant number 12/RC/2275], co-funded under the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelleher, J.F., Madi, A.M., Gilvary, G.C. et al. Metformin Hydrochloride and Sitagliptin Phosphate Fixed-Dose Combination Product Prepared Using Melt Granulation Continuous Processing Technology. AAPS PharmSciTech 21, 23 (2020). https://doi.org/10.1208/s12249-019-1553-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1553-2