Abstract
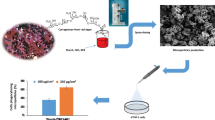
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis and represents one of the leading causes of mortality worldwide due to multidrug-resistant TB (MDR-TB). In our work, a new formulation of biodegradable PLGA microparticles was developed for pulmonary administration of gatifloxacin, using a surface modifier agent to actively target alveolar macrophages thereby allowing to gain access of the drug to Mycobacterium tuberculosis. For this, rapid uptake of the particles by macrophages is beneficial. This process was evaluated with fluorescein-loaded microparticles using PLGA 502 or PLGA 502H as polymers and labrafil as surface modifier. Cell phagocytosis was studied in raw 264.7 mouse macrophage cell line after 3, 5, 24, and 48 h incubation with the microparticles. Labrafil enhanced the uptake rate of PLGA 502H microparticles by macrophages which was directly related to the modification of the polymer matrix. Gatifloxacin-loaded PLGA microparticles using PLGA 502 or PLGA 502H and labrafil were prepared. From our results, only microparticles prepared with PLGA 502H and labrafil exhibited high encapsulation efficiency (89.6 ± 0.2%), rapid phagocytosis by macrophages (3 h), and remained inside the cells for at least 48 h, thereby resulting in a suitable carrier to potentially treat MDR-TB.








Similar content being viewed by others
References
WHO. Global tuberculosis report 2018 in http://www.who.int. 2018. Last accessed June 2018.
Lin SY, Desmond EP. Molecular diagnosis of tuberculosis and drug resistance. Clin Lab Med. 2014;34:297–314. https://doi.org/10.1016/j.cll.2014.02.005.
Salamon H, Yamaguchi KD, Cirillo DM, et al. Integration of published information into a resistance-associated mutation database for Mycobacterium tuberculosis. J Infect Dis. 2015;2:50–7. https://doi.org/10.1093/infdis/jiu816.
Hirota K, Terada H. Endocytosis of particles formulations by macrophages and its application to clinical treatment. INTECH, Open Access Publisher. 2012;16:13–428. https://doi.org/10.5772/45820.
Patel B, Gupta N, Ahsan F. Particle engineering to enhance of lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur J Pharm Biopharm. 2015;89:163–74. https://doi.org/10.1016/j.ejpb.2014.12.001.
Hirota K, Hasegawa T, Nakajima T, Makino K, Terada H. Phagostimulatory effect of uptake of PLGA microspheres loaded with rifampicin on alveolar macrophages. Colloids Surf B: Biointerfaces. 2011;87:293–8. https://doi.org/10.1016/j.colsurfb.2011.05.032.
Sharma R, Muttil P, Yadav AB, et al. Uptake of inhalable microparticles affects defense responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J Antimicrob Chemother. 2007;59:499–506. https://doi.org/10.1093/jac/dkl533.
Muttil P, Kaur J, Kumar K, Yadav AB, Sharma R, Misra A. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur J Pharm Sci. 2007;32:140–50. https://doi.org/10.1016/j.ejps.2007.06.006.
Rustomjee R, Lienhardt C, Kanyok T, et al. A phase II study of the sterilizing activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;2:128–38.
Merle CS, Sismanidis C, Sow OB, et al. A pivotal registration phase III, multicenter, randomized tuberculosis controlled trial: design issues and lesson learnt from the gatifloxacin for TB (OFLOTUB) project. Trials. 2012;18:13–61. https://doi.org/10.1186/1745-6215-13-61.
Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin containing regimen for treating tuberculosis. N Engl J Med. 2014;17:1588–98. https://doi.org/10.1056/NEJMoa1315817.
Ruan Q, Liu Q, Sun F, et al. Moxifloxacin and gatifloxacin for initial therapy of tuberculosis: a meta-analysis of randomized clinical trials. Emerg Microbes Infect. 2016;24:1038–50. https://doi.org/10.1038/emi.2016.12.
European Directorate for Quality in Medicines (EDQM). European Pharmacopeia 9th Edition (9.0). Preparations for inhalations: aerodynamic assessment of fine particles. Strasburg: France EDQM; 2016.
Fernandez-Carballidoa A, Pastoriza P, Barcia E, et al. PLGA/PEG-derivative polymeric matrix for drug delivery systemapplications: characterization and cell viability studies. Int J Pharm. 2008;352:50–7. https://doi.org/10.1016/j.ijpharm.2007.10.007.
Puebla P, Pastoriza P, Barcia E, Fernández-Carballido A. PEG-derivative effectively modifies the characteristics of indomethacin-PLGA microspheres destined to intra-articular administration. J Microencapsul. 2005;22:793–808. https://doi.org/10.1080/02652040500273902.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97. https://doi.org/10.1146/annurev.immunol.021908.132620.
Russel DG, Cardona PJ, Kim MJ, et al. F. Foamy macrophages and the progression of the human TB granuloma. Nat. Immunol. 2009;10:943–8. https://doi.org/10.1038/ni.1781.
Pacheco P, White D, Sulchek T. Effects of microparticle size and Fc density on macrophage phagocytosis. PLoS One. 2013;8:e60989. https://doi.org/10.1371/journal.pone.0060989.
Chono S, Tanino T, Seki T, Morimoto K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J Drug Target. 2006;14:557–66. https://doi.org/10.1080/10611860600834375.
Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, et al. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release. 2007;119:69–76. https://doi.org/10.1016/j.jconrel.2007.01.013.
Simón-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ. PEGylated-PLGA microparticles containing VEGF for long term drug delivery. Int J Pharm. 2012;440:13–8. https://doi.org/10.1016/j.ijpharm.2012.07.006.
Mathaes R, Winter G, Besheer A, et al. Influence of particle geometry and PEGylation on phagocytosis of particulate carriers. Int J Pharm. 2014;465:1–6. https://doi.org/10.1016/j.ijpharm.2014.02.037.
Champion S, Mitragotri A. Role of target geometry in phagocytosis. Proc Natl Acad Sci. 2006;103:4930–4. https://doi.org/10.1073/pnas.0600997103.
Ahsan F, Rivas IP, Khan MA, et al. Targeting to macrophages: role of physicochemical properties of particulate carriers liposomes and microspheres on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. https://doi.org/10.1016/S0168-3659(01)00549-1.
Verhoef J, Anchordoquy TJ. Questioning the use of PEGylation for drug delivery. Drug Deliv Transl Res. 2003;3:499–503.
Yang C, Gao S, Dagnæs-Hansen F, Jakobsen M, Kjems J. Impact of PEG chain length on the physical properties and bioactivity of PEGylated chitosan/ siRNA nanoparticles in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9:12203–16. https://doi.org/10.1021/acsami.6b16556.
Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. https://doi.org/10.1016/j.ijpharm.2005.10.010.
Mosqueira C, Legrand P, Morgat J, et al. Biodistribution of long-circulating PEG-grafted nanocapsules in mice: effects of PEG chain length and density. Pharm Res. 2001;18:1411–9.
Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement and Fc receptor mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–37.
Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–45. https://doi.org/10.1016/S0952-7915(01)00309-0.
Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50:1209–27.
Gardner DL, Tweedle DEF. Pathology for surgeons in training. An A-Z Revision Text. 3th ed. Boca Raton: Taylor & Francis Group; 2002. p. 32.
Fernández A, Casan P. Deposition of inhaled particles in the lungs. Arch Bronconeumol. 2012;48:240–6. https://doi.org/10.1016/j.arbr.2012.02.006.
Acknowledgments
The authors wish to express their gratitude to Isabel Trabado from the Unidad de Cultivos de Células Humanas (Universidad de Alcalá, Spain) for her technical assistance.
Funding
This work was partially supported by the Complutense University of Madrid, UCM Research group 910.939.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal procedures were conducted according to the European Community Council Directive (010/63/UE).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marcianes, P., Negro, S., Barcia, E. et al. Potential Active Targeting of Gatifloxacin to Macrophages by Means of Surface-Modified PLGA Microparticles Destined to Treat Tuberculosis. AAPS PharmSciTech 21, 15 (2020). https://doi.org/10.1208/s12249-019-1552-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1552-3