Abstract
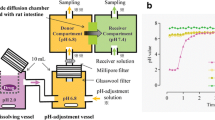
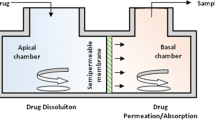
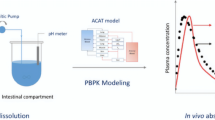
Combined dissolution and permeation systems are designed to simultaneously assess the dissolution of a pharmaceutical dosage form and the permeation of dissolved drugs therefrom. However, there were still some limitations on predicting the possible absorption rate–limiting steps and improving the in vitro–in vivo correlation (IVIVC) of a complete dosage form. In this study, the modified biorelevant media with some solubilizers and pH modifiers were integrated into the drug dissolution/absorption simulating system (DDASS). Indapamide, a poorly soluble compound (pKa = 8.8), was selected to validate the applicability of the modified biorelevant media. The elution and permeation dynamics of indapamide were investigated by using appropriate solubilizing agents in the DDASS. The absorption behaviors were analyzed after oral administration of indapamide in beagle dogs. The absorption rate–limiting steps and IVIVCs were predicted from the dissolution–permeation–absorption dynamic parameters. As a result, the absorption fraction of indapamide in the FaSSIFmod of DDASS was estimated to be approximately 100%, in accordance with its high permeability. The ratios of permeation rate to elution rate were 2.55 and 3.34 for the immediate- and sustained-release tablets of indapamide, respectively, suggesting a dissolution rate–limiting absorption for indapamine. In addition, point-to-point correlations were established between in vitro elution and in vivo absorption by the nonlinear and linear regression analysis ways (r > 0.85). The findings indicate that DDASS is a promising technique to develop improved IVIVCs of a complete dosage form, and the FaSSIFmod is suitable to predict the possible absorption rate–limiting steps of poorly soluble drugs in DDASS.








Similar content being viewed by others
References
Guerra A, Etienne-Mesmin L, Livrelli V, Denis S, Blanquet-Diot S, Alric M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012;30:591–600. https://doi.org/10.1016/j.tibtech.2012.08.001.
Cascone S, De SF, Lamberti G, Titomanlio G. The influence of dissolution conditions on the drug ADME phenomena. Eur J Pharm Biopharm. 2011;79:382–91. https://doi.org/10.1016/j.ejpb.2011.04.003.
Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, et al. Impact of solubilizing additives on supersaturation and membrane transport of drugs. Pharm Res. 2015;32:3350–64. https://doi.org/10.1007/s11095-015-1712-4.
Borbás E, Nagy ZK, Nagy B, Balogh A, Farkas B, Tsinman O, et al. The effect of formulation additives on in vitro dissolution-absorption profile and in vivo bioavailability of telmisartan from brand and generic formulations. Eur J Pharm Sci. 2018;114:310–7. https://doi.org/10.1016/j.ejps.2017.12.029.
Dahan A, Miller JM. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14:244–51. https://doi.org/10.1208/s12248-012-9337-6.
Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility-permeability interplay when using cosolvents for solubilization: revising the way we use solubility-enabling formulations. Mol Pharm. 2012;9:581–90. https://doi.org/10.1021/mp200460u.
Beig A, Fine-Shamir N, Lindley D, Miller JM, Dahan A. Advantageous solubility-permeability interplay when using amorphous solid dispersion (ASD) formulation for the BCS class IV P-gp substrate rifaximin: simultaneous increase of both the solubility and the permeability. AAPS J. 2017;19:806–13. https://doi.org/10.1208/s12248-017-0052-1.
Ginski MJ, Taneja R, Polli JE. Prediction of dissolution-absorption relationships from a continuous dissolution/Caco-2 system. AAPS Pharm Sci. 1999;1:27–38. https://doi.org/10.1016/S0378-5173(98)00330-5.
Kobayashi M, Sada N, Sugawara M, Iseki K, Miyazaki K. Development of a new system for prediction of drug absorption that takes into account drug dissolution and pH change in the gastro-intestinal tract. Int J Pharm. 2001;221:87–94. https://doi.org/10.1016/S0378-5173(01)00663-9.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20:1674–80. https://doi.org/10.1023/A:1026107906191.
Motz SA, Schaefer UF, Balbach S, Eichinger T, Lehr CM. Permeability assessment for solid oral drug formulations based on Caco-2 monolayer in combination with a flow through dissolution cell. Eur J Pharm Biopharm. 2007;66:286–95. https://doi.org/10.1016/j.ejpb.2006.10.015.
Li Z, He X. Physiologically based in vitro models to predict the oral dissolution and absorption of a solid drug delivery system. Curr Drug Metab. 2015;16:777–806. https://doi.org/10.2174/1389200216666150812123836.
Buch P, Langguth P, Kataoka M, Yamashita S. IVIVC in oral absorption for fenofibrate immediate release tablets using a dissolution/permeation system. J Pharm Sci. 2009;98:2001–9. https://doi.org/10.1002/jps.21576.
Buch P, Holm P, Thomassen JQ, Scherer D, Branscheid R, Kolb U, et al. IVIVC for fenofibrate immediate release tablets using solubility and permeability as in vitro predictors for pharmacokinetics. J Pharm Sci. 2010;99:4427–36. https://doi.org/10.1002/jps.22148.
Li ZQ, He X, Gao X, Xu YY, Wang YF, Gu H, et al. Study on dissolution and absorption of four dosage forms of isosorbide mononitrate: level A in vitro-in vivo correlation. Eur J Pharm Biopharm. 2011;79:364–71. https://doi.org/10.1016/j.ejpb.2011.04.015.
Li ZQ, Tian S, Gu H, Wu ZG, Nyagblordzro M, Feng G, et al. In vitro-in vivo predictive dissolution-permeation-absorption dynamics of highly permeable drug extended-release tablets via drug dissolution/absorption simulating system and pH alteration. AAPS PharmSciTech. 2018;19:1–12. https://doi.org/10.1208/s12249-018-0996-1.
Wojnarowska Z, Grzybowska K, Hawelek L, Dulski M, Wrzalik R, Gruszka I, et al. Molecular dynamics, physical stability and solubility advantage from amorphous indapamide drug. Mol Pharm. 2013;10:3612–27. https://doi.org/10.1021/mp400116q.
Stetinova V, Kubant P, Svoboda Z, Kopecky J, Smetanova L, Kvetina J. In vitro and in vivo prediction of indapamide gastrointestinal absorption. Toxicol Lett. 2007;172:S56-S56. https://doi.org/10.1016/j.toxlet.2007.05.171.
Caruso FS, Szabadi RR, Vukovich RA. Pharmacokinetics and clinical pharmacology of indapamide. Am Heart J. 1983;106:212–20. https://doi.org/10.1016/0002-8703(83)90119-9.
Damien G, Huet DBB, Schiavi P. Galenic development and pharmacokinetic profile of indapamide sustained release 1.5 mg. Clin Pharmacokinet. 1999;37:13–9. https://doi.org/10.2165/00003088-199937001-00003.
Polli JE, Crison JR, Amidon GL. Novel approach to the analysis of in vitro-in vivo relationships. J Pharm Sci. 1996;85:753–60. https://doi.org/10.1021/js9503587.
Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. https://doi.org/10.1016/0378-5173(83)90064-9.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. https://doi.org/10.1002/jps.2600521210.
Duizer E, Penninks AH. Comparison of permeability characteristics of the human colonic Caco-2 and rat small intestinal IEC18 cell lines. J Control Release. 1997;49:39–49. https://doi.org/10.1016/S0168-3659(97)00058-8.
Imai T, Sakai M, Ohtake H, Azuma H, Otagiri M. In vitro and in vivo evaluation of the enhancing activity of glycyrrhizin on the intestinal absorption of drugs. Pharm Res. 1999;16:80–6. https://doi.org/10.1023/A:1018822829302.
He X, Sugawara M, Kobayashi M, Takekuma Y, Miyazaki K. An in vitro system for prediction of oral absorption of relatively water-soluble drugs and ester prodrugs. Int J Pharm. 2003;263:35–44. https://doi.org/10.1016/S0378-5173(03)00343-0.
He X, Sugawara M, Takekuma Y, Miyazaki K. Absorption of ester prodrugs in Caco-2 and Rat intestine models. Antimicrob Agents Chemother. 2004;48:2604–9. https://doi.org/10.1128/AAC.48.7.2604-2609.2004.
Kataoka M, Yano K, Hamatsu Y, Masaoka Y, Sakuma S, Yamashita S. Assessment of absorption potential of poorly water-soluble drugs by using the dissolution/permeation system. Eur J Pharm Biopharm. 2013;85(3):1317–24. https://doi.org/10.1016/j.ejpb.2013.06.018.
Kataoka M, Fukahori M, Ikemura A, Kubota A, Higashino H, Sakuma S, et al. Effects of gastric ph on oral drug absorption: in vitro assessment using a dissolution/permeation system reflecting the gastric dissolution process. Eur J Pharm Biopharm. 2016;101:103–11. https://doi.org/10.1016/j.ejpb.2016.02.002.
Mizoguchi M, Kataoka M, Yokoyama K, Aihara R, Wada K, Yamashita S. Application of an in vitro dissolution/permeation system to early screening of oral formulations of poorly soluble, weakly basic drugs containing an acidic pH-modifier. J Pharm Sci. 2018;107:2404–10. https://doi.org/10.1016/j.xphs.2018.05.009.
Patel N, Forbes B, Eskola S, Murray J. Use of simulated intestinal fluids with Caco-2 cells and rat ileum. Drug Dev Ind Pharm. 2006;32(2):151–61. https://doi.org/10.1080/03639040500465991.
Kristin F, Carl R, David D, Filippos K, Moritz AK, Johanna M, et al. Optimization of the Ussing chamber setup with excised rat intestinal segments for dissolution/permeation experiments of poorly soluble drugs. Drug Dev Ind Pharm. 2017;43:338–46. https://doi.org/10.1080/03639045.2016.1251449.
Borbás E, Balogh A, Bocz K, Müller J, Kiserdei É, Vigh T, et al. In vitro dissolution–permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFlux™. Int J Pharm. 2015;491:180–9. https://doi.org/10.1016/j.ijpharm.2015.06.019.
Liu WJ, He X, Li ZQ, Gao XM, Ma YT, Xun MJ, et al. Development of a bionic system for the simultaneous prediction of the release/absorption characteristics of enteric-coated formulations. Pharm Res. 2013;30:596–605. https://doi.org/10.1007/s11095-012-0905-3.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–405. https://doi.org/10.1021/mp100149j.
Song W, Cun D, Quan P, Liu N, Chen Y, Cui H, et al. Dual-directional regulation of drug permeating amount by combining the technique of ion-pair complexation with chemical enhancers for the synchronous permeation of indapamide and bisoprolol in their compound patch through rabbit skin. Eur J Pharm Biopharm. 2015;91:59–65. https://doi.org/10.1016/j.ejpb.2015.01.025.
Yaro P, He X, Liu W, Xun M, Ma Y, Li Z, et al. In vitro-in vivo correlations for three different commercial immediate-release indapamide tablets. Drug Dev Ind Pharm. 2014;40:1670–6. https://doi.org/10.3109/03639045.2013.842577.
Robinson DM, Wellington K. Indapamide sustained release. Drugs. 2006;66(2):257–71. https://doi.org/10.2165/00003495-200666020-00011.
Cuine A, De Barochez BH, Guez D. Matrix tablet permitting the sustained release of indapamide after oral administration: U.S. Patent 5,334,392[P]. 1994-8-2.
Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89(4):429–42. https://doi.org/10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J.
Berginc K, Trontelj J, Kristl A. Bio-relevant media to assess drug permeability: sodium taurocholate and lecithin combination or crude bile? Int J pharm. 2012;429(1-2):22–30. https://doi.org/10.1016/j.ijpharm.2012.03.015.
Sugano K, Kataoka M, da Costa MC, Yamashita S. Prediction of food effect by bile micelles on oral drug absorption considering free fraction in intestinal fluid. Eur JPharm Sci. 2010;40:118–24. https://doi.org/10.1016/j.ejps.2010.03.011.
Buckley ST, Fischer SM, Fricker G, Brandl M. In vitro models to evaluate the permeability of poorly soluble drug entities: challenges and perspectives. Eur J Pharm Sci. 2012;45(3):235–50. https://doi.org/10.1016/j.ejps.2011.12.007.
Saha P, Kou JH. Effect of solubilizing excipients on permeation of poorly water-soluble compounds across Caco-2 cell monolayers. Eur J Pharm Biopharm. 2000;50(3):403–11. https://doi.org/10.1016/S0939-6411(00)00113-2.
Collnot E-M, Baldes C, Wempe MF, Hyatt J, Navarro L, Edgar KJ, et al. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Controlled Release. 2006;111:35–40. https://doi.org/10.1016/j.jconrel.2005.11.005.
Lind ML, Jacobsen J, Holm R, Müllertz A. Development of simulated intestinal fluids containing nutrients as transport media in the Caco-2 cell culture model: assessment of cell viability, monolayer integrity and transport of a poorly aqueous soluble drug and a substrate of efflux mechanisms. Eur J Pharm Sci. 2007;32(4-5):261–70. https://doi.org/10.1016/j.ejps.2007.08.002.
Campbell DB, Taylor AR, Hopkins YW, Williams JRB. Pharmacokinetics and metabolism of indapamide: a review. Curr Med Res Opin. 2008;5:13–24. https://doi.org/10.1185/03007997709110219.
Schiavi P, Jochemsen R, Guez D. Pharmacokinetics of sustained and immediate release formulations of indapamide after single and repeated oral administration in healthy volunteers. Fund Clin Pharmacol. 2010;14:139–46. https://doi.org/10.1111/j.1472-8206.2000.tb00402.x.
Sassard J, Bataillard A, McIntyre H. An overview of the pharmacology and clinical efficacy of indapamide sustained release. Fund Clin Pharmacol. 2005;19(6):637–45. https://doi.org/10.1111/j.1472-8206.2005.00377.x.
Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–80. https://doi.org/10.1002/bdd.2510160502.
Sutton SC. Companion animal physiology and dosage form performance. Adv Drug Deliv Rev. 2004;56(10):1383–98. https://doi.org/10.1016/j.addr.2004.02.013.
Ayman E-K, Hurst S, Brodfuehrer J, Cho-Ming L. Anatomical and physiological factors affecting oral drug bioavailability in rats, dogs, and humans. In: Oral bioavailability: basic principles, advanced concepts, and applications. Hoboken: John Wiley & Sons, Inc; 2011.
Funding
This work was supported by grants from the Key Support Projects of Tianjin Science and Technology (16YFZCSY00440), Changjiang Scholars and Innovative Research Team (IRT_14R41), and Tianjin Natural Science Foundation (18JCQNJC83800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study protocol was approved by the Ethical Review Committee of Second Affiliated Hospital of Tianjin University of TCM. The animal experiments were performed in accordance with the Principles of Laboratory Animal Care (NIH No. 8523).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 402 kb)
Rights and permissions
About this article
Cite this article
Li, Z., He, X., Tian, S. et al. Simultaneous Evaluation of Dissolution and Permeation of Oral Drug Solid Formulations for Predicting Absorption Rate–Limiting Factors and In Vitro–In Vivo Correlations: Case Study Using a Poorly Soluble Weakly Basic Drug. AAPS PharmSciTech 20, 321 (2019). https://doi.org/10.1208/s12249-019-1544-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1544-3