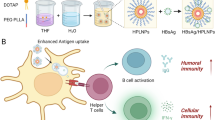
Abstract
The launched hepatitis B vaccine could induce powerful antibodies, whereas it failed to improve potent cellular immune responses due to that the Th2-type response-induced aluminum adjuvant was adopted. Here, to target antigen-presenting cells under the epidermis and induce potent cellular and humoral immune responses, mannose-modified poly d,l-lactide-co-glycolic acid (PLGA) was synthesized and nanoparticle (MNP)-loaded hepatitis B surface antigen (HBsAg) protein was prepared. HBsAg could be slowly released and highly presented to lymphocytes which facilitated to produce long-lasting immunity based on characters of PLGA. In vitro uptake test results showed that MNPs could enhance internalization in bone marrow–derived dendritic cells (BMDCs) and RAW 264.7 cells. Subcutaneous delivery of MNPs into mice kept humoral immune and strengthened cellular immune responses. Experimental results indicated that MNPs showed significantly modified properties compared with parental PLGA nanoparticles. Thus, the obtained MNPs could be a promising vehicle for hepatitis B vaccine delivery.





Similar content being viewed by others
References
Salpini R, Colagrossi L, Bellocchi M, Surdo M, et al. Hepatitis B surface antigen genetic elements critical for immune escape correlate with hepatitis B virus reactivation upon immunosuppression. Hepatology. 2015;61(3):823–33.
Zerbini A, Pilli M, Boni C, Fisicaro P, Penna A, di Vincenzo P, et al. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008;134(5):1470–81.
Uto T, Akagi T, Toyama M, Nishi Y, Shima F, Akashi M, et al. Comparative activity of biodegradable nanoparticles with aluminum adjuvants: antigen uptake by dendritic cells and induction of immune response in mice. Immunol Lett. 2011;140(1–2):36–43.
Tomljenovic L, Shaw CA. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus. 2012;21:223–30.
Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–7.
Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26(10):1375–86.
Griffin DE. The immune response in measles: virus control, clearance and protective immunity. Viruses-Basel. 2016;8(10):8.
Ford Versypt AN, Pack DW, Braatz RD. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres--a review. Journal of Controlled Release Official Journal of the Controlled Release Society. 2013;165(1):29–37.
Ahmad E, Zia Q, Fatima MT, Owais M, Saleemuddin M. Vaccine potential of plasma bead-based dual antigen delivery system against experimental murine candidiasis. Int J Biol Macromol. 2015;81:100–11.
Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–22.
Cruz LJ, Tacken PJ, Eich C, Rueda F, Torensma R, Figdor CG. Controlled release of antigen and Toll-like receptor ligands from PLGA nanoparticles enhances immunogenicity. Nanomedicine. 2017;12(5):491–510.
Salvador A, Sandgren KJ, Liang F, Thompson EA, Koup RA, Pedraz JL, et al. Design and evaluation of surface and adjuvant modified PLGA microspheres for uptake by dendritic cells to improve vaccine responses. Int J Pharm. 2015;496(2):371–81.
Wang S, Shao M, Zhong Z, Wang A, Cao J, Lu Y, et al. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Delivery. 2017;24(1):1791–800.
Mansouri A, Abnous K, Alibolandi M, Taghdisi SM, Ramezani M. Targeted delivery of tacrolimus to T cells by pH-responsive aptamer-chitosan- poly(lactic-co-glycolic acid) nanocomplex. J Cell Physiol. 2019.
Platel A, Carpentier R, Elodie B, et al. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J Appl Toxicol. 2016;36:434–44.
Dharmendra R, Vivek M, Suresh MR, Kamaljit K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine. 2012;30(50):7292–9.
Rajaram MVS, Arnett E, Azad AK, Guirado E, Ni B, Gerberick AD, et al. M. tuberculosis-initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcR gamma-chain, Grb2, and SHP-1. Cell Rep. 2017;21(1):126–40.
Chen YZ, Ruan GX, Yao XL, Li LM, Hu Y, Tabata Y, et al. Co-transfection gene delivery of dendritic cells induced effective lymph node targeting and anti-tumor vaccination. Pharm Res. 2013;30(6):1502–12.
Gennari A, Pelliccia M, Donno R, Kimber I, Tirelli N. Mannosylation allows for synergic (CD44/C-type lectin) uptake of hyaluronic acid nanoparticles in dendritic cells, but only upon correct ligand presentation. Adv Healthc Mater. 2016;5(8):966–76.
Cai-Hong Z, Jian-Qing G, Ye-Ping Z, Wen-Quan L. A protein delivery system: biodegradable alginate-chitosan-poly(lactic-co-glycolic acid) composite microspheres. Biochem Biophys Res Commun. 2004;323(4):1321–7.
Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1(12):1297–302.
Zhou P, An T, Zhao C, Li Y, Li R, Yang R, et al. Lactosylated PLGA nanoparticles containing e-polylysine for the sustained release and liver-targeted delivery of the negatively charged proteins. Int J Pharm. 2015;478:633–43.
Maria AS, Anne DR, Virginie F, Bruno S, Araceli D, Carmen E, et al. Development of PLGA-mannosamine nanoparticles as oral protein carriers. Biomacromolecules. 2013;14(11):4046–52.
Kim HK, Park TG. Comparative study on sustained release of human growth hormone from semi-crystalline poly(L-lactic acid) and amorphous poly(D,L-lactic-co-glycolic acid) microspheres: morphological effect on protein release. J Control Release. 2004;98:115–25.
Costabile G, Gasteyer KI, Nadithe V, Van Denburgh K, Lin Q, Sharma S, et al. Physicochemical and in vitro evaluation of drug delivery of an antibacterial synthetic benzophenone in biodegradable PLGA nanoparticles. AAPS PharmSciTech. 2018;19(8):3561–70.
Dewangan HK, Singh S, Maurya L, Srivastava A. Hepatitis B antigen loaded biodegradable polymeric nanoparticles: formulation optimization and in-vivo immunization in BALB/c mice. Curr Drug Deliv. 2018;15(8):1204–15.
Thomas C, Rawat A, Hope-Weeks L, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8(2):405–15.
Edelman R, Kusner I, Kisiliak R, Srebnik S, Livney YD. Sugar stereochemistry effects on water structure and on protein stability: the templating concept. Food Hydrocoll. 2015;48:27–37.
Walters AA, Somaravarapu S, Riitho V, Stewart GR, Charleston B, Steinbach F, et al. Assessment of the enhancement of PLGA nanoparticle uptake by dendritic cells through the addition of natural receptor ligands and monoclonal antibody. Vaccine. 2015;33(48):6588–95.
Liu Y, Yin Y, Wang LY, Zhang WF, Chen XM, Yang XX, et al. Surface hydrophobicity of microparticles modulates adjuvanticity. J Mater Chem B. 2013;1(32):3888–96.
Claire D, Maurizia B, Gary R, Theodoros C, Kennedy PT, Pietro L, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204(3):667–80.
Acknowledgments
We thank Gillian Campbell, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This study was supported by Science and Technology Department of Zhejiang province public welfare technology research project (Grant No. 2015C31100), Key Laboratory of Ningbo, China (No. 2016A22002), and National Natural Science Foundation of China (Grant Nos. 81173000, 81873838).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
The authors alone are responsible for the content and writing of this article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Animal experiments were approved by the experimental animal ethics committee of Zhejiang University (ZJU20160200) in the animal experimentation section.
Rights and permissions
About this article
Cite this article
Zhu, J., Qin, F., Ji, Z. et al. Mannose-Modified PLGA Nanoparticles for Sustained and Targeted Delivery in Hepatitis B Virus Immunoprophylaxis. AAPS PharmSciTech 21, 13 (2020). https://doi.org/10.1208/s12249-019-1526-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1526-5