Abstract
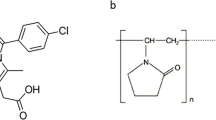
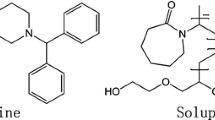
With the increase concern of solubilization for insoluble drug, ternary solid dispersion (SD) formulations developed more rapidly than binary systems. However, rational formulation design of ternary systems and their dissolution molecular mechanism were still under development. Current research aimed to develop the effective ternary formulations and investigate their molecular mechanism by integrated experimental and modeling techniques. Glipizide (GLI) was selected as the model drug and PEG was used as the solubilizing polymer, while surfactants (e.g., SDS or Tween80) were the third components. SD samples were prepared at different weight ratio by melting method. In the dissolution tests, the solubilization effect of ternary system with very small amount of surfactant (drug/PEG/surfactant 1/1/0.02) was similar with that of binary systems with high polymer ratios (drug/PEG 1/3 and 1/9). The molecular structure of ternary systems was characterized by differential scanning calorimetry (DSC), infrared absorption spectroscopy (IR), X-ray diffraction (XRD), and scanning electron microscope (SEM). Moreover, molecular dynamic (MD) simulations mimicked the preparation process of SDs, and molecular motion in solvent revealed the dissolution mechanism of SD. As the Gordon–Taylor equation described, the experimental and calculated values of Tg were compared for ternary and binary systems, which confirmed good miscibility of GLI with other components. In summary, ternary SD systems could significantly decrease the usage of polymers than binary system. Molecular mechanism of dissolution for both binary and ternary solid dispersions was revealed by combined experiments and molecular modeling techniques. Our research provides a novel pathway for the further research of ternary solid dispersion formulations.











Similar content being viewed by others
References
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Renuka, Singh SK, Gulati M, Narang R. Stable amorphous binary systems of glipizide and atorvastatin powders with enhanced dissolution profiles: formulation and characterization. Pharm Dev Technol. 2017;22(1):13–25.
Löbenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Food, Administration D. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. Food and Drug Administration, Rockville, MD. 2000.
Sun DD, Lee PI. Evolution of supersaturation of amorphous pharmaceuticals: the effect of rate of supersaturation generation. Mol Pharm. 2013;10(11):4330–46.
Verma S, Rudraraju VS. Wetting kinetics: an alternative approach towards understanding the enhanced dissolution rate for amorphous solid dispersion of a poorly soluble drug. AAPS PharmSciTech. 2015;16(5):1079–90.
Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3):278–87.
Baghel S, Cathcart H, O’Reilly NJ. Investigation into the solid-state properties and dissolution profile of spray-dried ternary amorphous solid dispersions: a rational step toward the design and development of a multicomponent amorphous system. Mol Pharm. 2018;15:3796–812.
Park J-B, Park C, Piao ZZ, Amin HH, Meghani NM, Tran PH, et al. pH-independent controlled release tablets containing nanonizing valsartan solid dispersions for less variable bioavailability in humans. J Drug Deliv Sci Tec. 2018;46:365–77.
Orlandi S, Priotti J, Diogo HP, Leonardi D, Salomon CJ, Nunes TG. Structural elucidation of Poloxamer 237 and Poloxamer 237/Praziquantel solid dispersions: impact of poly (Vinylpyrrolidone) over drug recrystallization and dissolution. AAPS PharmSciTech. 2018;19(3):1274–86.
Lehmkemper K, Kyeremateng SO, Degenhardt M, Sadowski G. Influence of low-molecular-weight excipients on the phase behavior of pvpva64 amorphous solid dispersions. Pharm Res. 2018;35(1):25.
Alhayali A, Tavellin S, Velaga S. Dissolution and precipitation behavior of ternary solid dispersions of ezetimibe in biorelevant media. Drug Dev Ind Pharm. 2017;43(1):79–88.
Deshpande TM, Shi H, Pietryka J, Hoag SW, Medek A. Investigation of polymer/surfactant interactions and their impact on Itraconazole solubility and precipitation kinetics for developing spray-dried amorphous solid dispersions. Mol Pharm. 2018;15(3):962–74.
Li M, Ioannidis N, Gogos C, Bilgili E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur J Pharm Biopharm. 2017;119:68–80.
Segale L, Giovannelli L, Mannina P, Pattarino F. Formulation and characterization study of itraconazole-loaded microparticles. Pharm Dev Technol. 2015;20(2):153–8.
Pongpeerapat A, Itoh K, Tozuka Y, Moribe K, Oguchi T, Yamamoto K. Formation and stability of drug nanoparticles obtained from drug/PVP/SDS ternary ground mixture. J Drug Deliv Sci Tec. 2004;14(6):441–7.
Rao JV, Pore YV, Shinde VR. Development and characterization of ternary solid dispersion systems of olmesartan medoxomil. Lat Am J Pharm 2011;30.
El Maghraby GM, Alomrani AH. Effect of binary and ternary solid dispersions on the in vitro dissolution and in situ rabbit intestinal absorption of gliclazide. Pak J Pharm Sci. 2011;24(4):459–68.
Zaki RM, Ali AA, El Menshawi SF, Bary AA. Effect of binary and ternary solid dispersions prepared by fusion method on the dissolution of poorly water soluble diacerein. Int J Drug Deliv. 2013;5(1):99–109.
Wang H, Xu HX, Zhang N, Hu LD, editors. Enhancement of Dissolution Rate of Daidzein in Ternary Solid Dispersions. Adv Mater Res; 2012: Trans Tech Publ.
Kadir MF, Sayeed MSB, Khan RI, Shams T, Islam MS. Study of binary and ternary solid dispersion of ibuprofen for the enhancement of oral bioavailability. JAPS. 2011;1(9):13.
Kadir MF, Alam MR, Rahman AB, Jhanker YM, Shams T, Khan RI. Study of binary and ternary solid dispersion of spironolactone prepared by co-precipitation method for the enhancement of Oral bioavailability. JAPS. 2012;2(10):117.
Li J, Liu P, Liu J-P, Zhang W-L, Yang J-K, Fan Y-Q. Novel Tanshinone II a ternary solid dispersion pellets prepared by a single-step technique: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80(2):426–32.
Jung HJ, Ahn HI, Park JY, Ho MJ, Lee DR, Cho HR, et al. Improved oral absorption of tacrolimus by a solid dispersion with hypromellose and sodium lauryl sulfate. Int J Biol Macromol. 2016;83:282–7.
Mura P, Moyano J, González-Rodríguez M, Rabasco-Alvarez A, Cirri M, Maestrelli F. Characterization and dissolution properties of ketoprofen in binary and ternary solid dispersions with polyethylene glycol and surfactants. Drug Dev Ind Pharm. 2005;31(4–5):425–34.
Goddeeris C, Willems T, Houthoofd K, Martens J, Van den Mooter G. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary solid dispersion with TPGS 1000 and Eudragit E100. Eur J Pharm Biopharm. 2008;70(3):861–8.
Cirri M, Maestrelli F, Corti G, Mura P, Valleri M. Fast-dissolving tablets of glyburide based on ternary solid dispersions with PEG 6000 and surfactants. Drug Deliv. 2007;14(4):247–55.
Janssens S, Nagels S, De Armas HN, D’autry W, Van Schepdael A, Van den Mooter G. Formulation and characterization of ternary solid dispersions made up of Itraconazole and two excipients, TPGS 1000 and PVPVA 64, that were selected based on a supersaturation screening study. Eur J Pharm Biopharm. 2008;69(1):158–66.
Goddeeris C, Willems T, Van den Mooter G. Formulation of fast disintegrating tablets of ternary solid dispersions consisting of TPGS 1000 and HPMC 2910 or PVPVA 64 to improve the dissolution of the anti-HIV drug UC 781. Eur J Pharm Sci. 2008;34(4–5):293–302.
Wang X, Michoel A, Van den Mooter G. Solid state characteristics of ternary solid dispersions composed of PVP VA64, Myrj 52 and itraconazole. Int J Pharm. 2005;303(1–2):54–61.
Mura P, Faucci M, Manderioli A, Bramanti G, Parrini P. Thermal behavior and dissolution properties of naproxen from binary and ternary solid dispersions. Drug Dev Ind Pharm. 1999;25(3):257–64.
Riekes MK, Engelen A, Appeltans B, Rombaut P, Stulzer HK, Van den Mooter G. New perspectives for fixed dose combinations of poorly water-soluble compounds: a case study with ezetimibe and lovastatin. Pharm Res. 2016;33(5):1259–75.
Bhise SD. Ternary solid dispersions of fenofibrate with poloxamer 188 and TPGS for enhancement of solubility and bioavailability. Int J Res Pharmaceut Biomed Sci. 2011;2(2):583–95.
Dave RH, Patel HH, Donahue E, Patel AD. To evaluate the change in release from solid dispersion using sodium lauryl sulfate and model drug sulfathiazole. Drug Dev Ind Pharm. 2013;39(10):1562–72.
Khaleel NY, Abdulrasool AA, Ghareeb MM, Hussain SA. Solubility and dissolution improvement of ketoprofen by solid dispersion in polymer and surfactant using solvent evaporation method. Acad Sci IJPPS. 2011;3:431–5.
Schlick T. Molecular modeling and simulation: an interdisciplinary guide: an interdisciplinary guide: Springer Science & Business Media; 2010.
De Vivo M, Masetti M, Bottegoni G, Cavalli A. Role of molecular dynamics and related methods in drug discovery. J Med Chem. 2016;59(9):4035–61.
Ouyang D, (Eds.) SCS. Computational Pharmaceutics: Application of Molecular Modeling in Drug Delivery: John Wiley & Sons Ltd., Chichester, West Sussex, United Kingdom, Hoboken(Book); 2015.
Ouyang D. Investigating the molecular structures of solid dispersions by the simulated annealing method. Chem Phys Lett. 2012;554:177–84.
Chan T, Ouyang D. Investigating the molecular dissolution process of binary solid dispersions by molecular dynamics simulations. AJPS. 2018;13(3):248–54.
Chen W, Ouyang D. Investigation of molecular dissolution mechanism of ketoprofen binary and ternary solid dispersions by molecular dynamics simulations. Mol Simul. 2017;43(13–16):1074–80.
Yale J-F. Oral antihyperglycemic agents and renal disease: new agents, new concepts. J Am Soc Nephrol. 2005;16(3 suppl 1):S7–S10.
Kirkpatrick S, Gelatt CD, Vecchi MP. Optimization by simulated annealing. Science. 1983;220(4598):671–80.
Kennedy M, Hu J, Gao P, Li L, Ali-Reynolds A, Chal B, et al. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Mol Pharm. 2008;5(6):981–93.
Gordon M, Taylor JS. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J Appl Chem. 1952;2(9):493–500.
Bliznyuk V, Assender H, Briggs G. Surface glass transition temperature of amorphous polymers. A new insight with SFM. Macromolecules. 2002;35(17):6613–22.
Boller A, Schick C, Wunderlich B. Modulated differential scanning calorimetry in the glass transition region. Thermochim Acta. 1995;266:97–111.
Abiad M, Carvajal M, Campanella O. A review on methods and theories to describe the glass transition phenomenon: applications in food and pharmaceutical products. Food Eng Rev. 2009;1(2):105–32.
Funding
Current research is financially supported by the University of Macau Research Grant (MYRG2016-00040-ICMS-QRCM) and the 2017 Sub-project 6 of National Major Scientific and Technological Special Project for “Significant New Drugs Development” of the Ministry of Science and Technology of China (2017ZX09101001006). Molecular modeling was performed in part at the High-Performance Computing Cluster (HPCC) which is supported by Information and Communication Technology Office (ICTO) of the University of Macau.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, R., Huang, T., Liu, X. et al. Insight into the Dissolution Molecular Mechanism of Ternary Solid Dispersions by Combined Experiments and Molecular Simulations. AAPS PharmSciTech 20, 274 (2019). https://doi.org/10.1208/s12249-019-1486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1486-9