Abstract
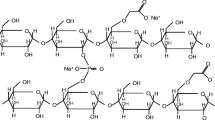
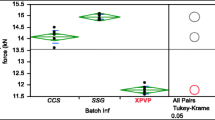
Here we investigated the disintegration action of the natural superdisintegrant soy polysaccharide (SP) and benchmarked it against sodium starch glycolate (SSG) and crospovidone (XPVP). Kinetics and mechanism of disintegration of various tablet formulations were monitored using a USB microscope connected to a computer, followed by image analysis. SP acts mainly by a swelling mechanism and it is most effective at concentrations of 4–8%. Its disintegration action is comparable with that of SSG and XPVP, in most cases. However, SP underperforms compared with these superdisintegrants, in extremely hard tablets containing a hydrophobic component. Moreover, it is more negatively affected by the concentration of magnesium stearate than SSG and XPVP. The disintegration action of SP is not affected by pH and ionic strength of the medium, but it is compromised by the presence of ethanol. This indicates that the concomitant administration of alcoholic beverages might hamper the disintegration of SP-containing tablets. Overall, SP is a promising tablet disintegrant for pharmaceutical and nutraceutical products.







Similar content being viewed by others
References
Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharm Res [Internet]. 2017/03/01. Springer US; 2017;34:890–917. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28251425.
Desai PM, Liew CV, Heng PWS. Review of disintegrants and the disintegration phenomena. J Pharm Sci [Internet] Elsevier; 2016;105:2545–55. Available from: https://doi.org/10.1016/j.xphs.2015.12.019.
Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol [Internet]. Taylor & Francis; 2016;21:763–74. Available from: https://doi.org/10.3109/10837450.2015.1045618.
JRS-Pharma. Emcosoy ® product literature provided by JRS Pharma GmBH and Co. Germany: Rosenberg; 2018.
Kalidindi SR, Shangraw RF. Evaluation of soy polysaccharide as a disintegrating agent part I: direct compression. Drug Dev Ind Pharm [Internet] Taylor & Francis; 1982;8:215–35. Available from: https://doi.org/10.3109/03639048209022099.
Malamataris S, Goidas P, Avgoustakis K. Comparative evaluation of soy polysaccharide as direct compression excipient. Drug Dev Ind Pharm [Internet]. Taylor & Francis; 1992;18:1575–87. Available from: https://doi.org/10.3109/03639049209040860.
Berardi A, Bisharat L, Blaibleh A, Pavoni L, Cespi M. A simple and inexpensive image analysis technique to study the effect of disintegrants concentration and diluents type on disintegration. J Pharm Sci [Internet] 2018;107:2643–52. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022354918303411
Quodbach J, Moussavi A, Tammer R, Frahm J, Kleinebudde P. Tablet disintegration studied by high-resolution real-time magnetic resonance imaging. J Pharm Sci [Internet]. Elsevier; 2014;103:249–55. Available from: https://doi.org/10.1002/jps.23789.
Desai PM, Liew CV, Heng PWS. Understanding disintegrant action by visualization. J Pharm Sci [Internet] Elsevier; 2012;101:2155–64. Available from: https://doi.org/10.1002/jps.23119
Berardi A, Bisharat L, AlKhatib HS, Cespi M. Zein as a pharmaceutical excipient in oral solid dosage forms: state of the art and future perspectives. AAPS PharmSciTech. 2018;19:2009–22.
Ursekar BM, Soni PS, Date AA, Nagarsenker MS. Characterization of soy polysaccharide and its in vitro and in vivo evaluation for application in colon drug delivery. AAPS PharmSciTech [Internet]. Springer US; 2012;13:934–43. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22739785
Fell JT, Newton JM. Determination of tablet strength by the diametral-compression test. J Pharm Sci. 1970;59:688–91.
Asare-Addo K, Conway BR, Larhrib H, Levina M, Rajabi-Siahboomi AR, Tetteh J, et al. The effect of pH and ionic strength of dissolution media on in-vitro release of two model drugs of different solubilities from HPMC matrices. Colloids Surfaces B Biointerfaces Elsevier. 2013;111:384–91.
Berardi A, Bisharat L, Cespi M, Basheti IA, Bonacucina G, Pavoni L, et al. Controlled release properties of zein powder filled into hard gelatin capsules. Powder Technol. 2017;320:703–13.
Bisharat L, AlKhatib HS, Muhaissen S, Quodbach J, Blaibleh A, Cespi M, et al. The influence of ethanol on superdisintegrants and on tablets disintegration. Eur J Pharm Sci [Internet]. 2019; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098719300120, 129, 140, 147
Papadimitriou E, Buckton G, Efentakis M. Probing the mechanisms of swelling of hydroxypropylmethylcellulose matrices. Int J Pharm [Internet]. 1993;98:57–62. Available from: http://www.sciencedirect.com/science/article/pii/037851739390041D
Chen YY, Hughes LP, Gladden LF, Mantle MD. Quantitative ultra-fast MRI of HPMC swelling and dissolution. J Pharm Sci [Internet]. Elsevier; 2010;99:3462–72. Available from: https://doi.org/10.1002/jps.22110.
Rajabi-Siahboomi AR, Bowtell RW, Mansfield P, Henderson A, Davies MC, Melia CD. Structure and behaviour in hydrophilic matrix sustained release dosage forms: 2. NMR-imaging studies of dimensional changes in the gel layer and core of HPMC tablets undergoing hydration. J Control Release [Internet]. 1994;31:121–8. Available from: http://www.sciencedirect.com/science/article/pii/0168365994000166
Ford JL. Design and evaluation of hydroxypropyl methylcellulose matrix tablets for oral controlled release: a historical perspective. Hydrophilic matrix tablets oral control release: Springer; 2014. p. 17–51.
Nokhodchi A, Rubinstein MH, Ford JL. The effect of particle size and viscosity grade on the compaction properties of hydroxypropylmethylcellulose 2208. Int J Pharm [Internet] 1995;126:189–97. Available from: http://www.sciencedirect.com/science/article/pii/0378517395041222.
Desai PM, Er PXH, Liew CV, Heng PWS. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech [Internet]. Springer US; 2014;15:1093–104. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24848762
Wang J, Wen H, Desai D. Lubrication in tablet formulations. Eur J Pharm Biopharm [Internet]. 2010;75:1–15. Available from: http://www.sciencedirect.com/science/article/pii/S0939641110000093
Cespi M, Bonacucina G, Roberts M, Hanson S, Jones S, Makevica E, et al. Evaluation of citrus fibers as a tablet excipient. AAPS PharmSciTech [Internet] Springer US; 2013;15:279–86. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24306677
Bolhuis GK, Van Kamp HV, Lerk CF, Sessink FGM. On the mechanism of action of modern disintegrants. Acta Pharm Technol. 1982;28:111–4.
Van Kamp H V, Bolhuis GK, De Boer AH, Lerk CF, Lie-A-Huen L. The role of water uptake on tablet disintegration. Design of an improved method for penetration measurements. Pharm Acta Helv 1986;61:22–29.
Khan KA, Rhodes CT. Water-sorption properties of tablet disintegrants. J Pharm Sci [Internet]. 1975;64:447–51. Available from: http://www.sciencedirect.com/science/article/pii/S0022354915400826
Ekmekciyan N, Tuglu T, El-Saleh F, Muehlenfeld C, Stoyanov E, Quodbach J. Competing for water: a new approach to understand disintegrant performance. Int J Pharm [Internet]. 2018;548:491–9. Available from: http://www.sciencedirect.com/science/article/pii/S0378517318304915
Strübing S, Metz H, Mäder K. Characterization of poly (vinyl acetate) based floating matrix tablets. J Control Release Elsevier. 2008;126:149–55.
Grund J, Koerber M, Walther M, Bodmeier R. The effect of polymer properties on direct compression and drug release from water-insoluble controlled release matrix tablets. Int J Pharm Elsevier. 2014;469:94–101.
Quodbach J, Kleinebudde P. Performance of tablet disintegrants: impact of storage conditions and relative tablet density. Pharm Dev Technol [Internet] Taylor & Francis; 2015;20:762–8. Available from: https://doi.org/10.3109/10837450.2014.920357
Chaheen M, Soulairol I, Bataille B, Yassine A, Belamie E, Sharkawi T. Chitin’s functionality as a novel disintegrant: benchmarking against commonly used disintegrants in different physicochemical environments. J Pharm Sci [Internet]. Elsevier; 2017;106:1839–48. Available from: https://doi.org/10.1016/j.xphs.2017.03.037.
Brannon-Peppas L, Peppas NA. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem Eng Sci [Internet]. 1991;46:715–22. Available from: http://www.sciencedirect.com/science/article/pii/000925099180177Z
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm [Internet] 2000;50:27–46. Available from: http://www.sciencedirect.com/science/article/pii/S0939641100000904
Siegel RA, Firestone BA. pH-dependent equilibrium swelling properties of hydrophobic polyelectrolyte copolymer gels. Macromolecules [Internet] American Chemical Society; 1988;21:3254–9. Available from: https://doi.org/10.1021/ma00189a021.
Caillard R, Remondetto GE, Mateescu MA, Subirade M. Characterization of amino cross-linked soy protein hydrogels. J Food Sci [Internet]. John Wiley & Sons, Ltd (10.1111); 2008;73:C283–91. Available from: https://doi.org/10.1111/j.1750-3841.2008.00780.x
Zhao N, Augsburger LL. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech [Internet]. Springer-Verlag; 2005;6:E120–6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16353956
Acknowledgements
The authors are grateful to Mr. Ra’fat Sweidan (Rimon Chemical, Jordan) and Mr. Benedikt Korona (JRS Pharma, Germany) for supplying the excipients used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 521 kb)
Rights and permissions
About this article
Cite this article
Amayreh, R., Bisharat, L., Cespi, M. et al. Evaluation of the Disintegration Action of Soy Polysaccharide by Image Analysis. AAPS PharmSciTech 20, 265 (2019). https://doi.org/10.1208/s12249-019-1477-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1477-x