Abstract
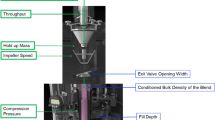
Continuous manufacturing (CM) has clear potential for manufacturing solid oral dosages. It provides several advantages that may aid the manufacturing and quality of drug products. However, one of the main challenges of this technology is the relatively large amount of knowledge required and the amounts of material needed to develop the process during the early stages of development. Early process development evaluations of continuous manufacturing equipment often require larger amounts of material compared with batch, which hinder CM prospect for drugs during the early stages of process development. In this work, a small-scale evaluation of the mixing process occurring in a continuous mixing system was performed. The evaluation involved the use of a small-scale “mixing cell” which was able to replicate the lubrication process of a continuous mixer. It is worth mentioning that we designed the mixing cell by reconfiguration of an existing continuous tubular blender. The extent of lubrication evaluation was performed for three example formulations and was done by mimicking the amount of shear provided to a formulation by means of matching the number of paddle-passes that a formulation experiences within a continuous blending process in the batch mixing cell. The evaluation showed that the small-scale mixing cell was able to replicate the extent of lubrication—evaluated by measuring the tensile strength of compacts being made with both the continuous and mixing cell experiments—occurring in the continuous mixer using a fraction of the amount of materials needed to perform the same evaluation in the continuous blending process.





Similar content being viewed by others
References
Editors P. Hovione plans commercial continuous manufacturing facility. PTSM: Pharmaceutical Technology Sourcing and Management. 2016;11.
Editors P. EMA approves Janssen drug made via continuous manufacturing. Editors Notes. 2017.
Editors, P. FDA to promote continuous manufacturing. 2017.
Editors P. Vertex receives FDA approval for continuously manufactured drug product. Editors Notes. 2018.
Gaspar F, Gil M, Matos N. Continuous processing: meeting the need for new manufacturing strategies. 2016.
Hausner D, Moore AC. Continuous manufacturing current status. Features. 2018.
Markarian J. Continuous manufacturing presses In: Equipment and Processing Report. 2017;10.
Pallone F. JrPallone & FDA commissioner Gottlieb visit Rutgers university to discuss innovative pharmaceutical manufacturing. House of representatives official website: frank Pallone, Jr Press Release. 2018.
Wang Y, Li T, Muzzio FJ, Glasser BJ. Predicting feeder performance based on material flow properties. Powder Technol. 2017;308:135–48.
Snickab V, Kumarc A, Verstraetend M, Pandelaerea K, Dhondtd J, DiPretorob G, et al. Impact of material properties and process variables on the residence time distribution in twin screw feeding equipment. Int J Pharm. 2019;556:200–16.
Bostijna N, Dhondta J, Ryckaerta A, Szabóa E, Dhondtb W, Van Snickcd B, et al. A multivariate approach to predict the volumetric and gravimetric feeding behavior of a low feed rate feeder based on raw material properties. Int J Pharm. 2019;557:342–53.
Escotet-Espinoza MS, Moghtadernejad S, Scicolone J, Wang Y, Pereira G, Schäfer E, et al. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018;339:659–76.
Muzzio FJ. Mixing mechanics: practical powder blending: micromixing, in Powder and Bulk Engineering. 2016.
Mehrotra A, Llusa M, Faqih A, Levin M, Muzzio FJ. Influence of shear intensity and total shear on properties of blends and tablets of lactose and cellulose lubricated with magnesium stearate. Int J Pharm. 2007;336(2):284–91.
Llusa M, Levin M, Snee RD, Muzzio FJ. Measuring the hydrophobicity of lubricated blends of pharmaceutical excipients. Powder Technol. 2010;198(1):101–7.
Portillo PM, Ierapetritou MG, Muzzio FJ. Effects of rotation rate, mixing angle, and cohesion in two continuous mixers—a statistical approach. Powder Technol. 2009;194:217–27.
Portillo PM, Vanarase AU, Ingram A, Seville JK. Investigation of the effect of impeller rotation rate, powder flowrate, and cohesion on powder flow behavior in a continuous blender using PEPT. Chem Eng Sci. 2010;65:5685–68.
Razavi S, Callegari G, Drazer G, Cuitino A. Toward predicting tensile strength of pharmaceutical tablets by ultrasound measurement in continuous manufacturing. Int J Pharm. 2016;507(1–2):83–9.
Li J, Wu Y. Lubricants in pharmaceutical solid dosage forms. Lubricants. 2014;2(1):21–43.
Vargas JM, Nielsen S, Cárdenas V, Gonzalez A, Aymat EY, Almodovar E, et al. Process analytical technology in continuous manufacturing of a commercial pharmaceutical product. Int J Pharm. 2018;538(1–2):167–78.
Robinson K. Continuous manufacturing: the facts and the future. Manuf Chem. 2019.
Gao Y, Vanarase A, Muzzio FJ, Ierapetritou M. Characterizing continuous powder mixing using residence time distribution. Chem Eng Sci. 2011;66(3):417–25.
Vanarase AU. A.F.J.M. Effect of operating conditions and design parameters in a continuous powder mixer. Powder Technol. 2011;208(1):26–36.
Escotet-Espinoza MS, Moghtadernejad S, Oka S, Wang Y, Roman-Ospino A, Schäfer E, et al. Effect of tracer material properties on the residence time distribution (RTD) of continuous powder blending operations. Part I of II: experimental evaluation. Powder Technol. 2019;342:744–63.
Escotet-Espinoza MS, Moghtadernejad S, Oka S, Wang Z, Wang Y, Roman-Ospino A, et al. Effect of material properties on the residence time distribution (RTD) characterization of powder blending unit operations. Part II of II: application of models. Powder Technol. 2019;344:525–44.
Oka S, Moghtadernejad S, Liu Z, Hausner D, Muzzio FJ. Lubrication in continuous tubular powder blenders. Pharm Technol. 2016;40(11):44–5.
Escotet-Espinoza MS, Vadodaria S, Singh R, Muzzio FJ, Ierapetritou MG. Modeling the effects of material properties on tablet compaction: a building block for controlling both batch and continuous pharmaceutical manufacturing processes. Int J Pharm. 2018;543(1):274–87.
Wang J, Wen H, Desai D. Lubrication in tablet formulations. Eur J Pharm Biopharm. 2010;75:1–15.
Miller TA, York P. Pharmaceutical tablet lubrication. Int J Pharm. 1988;41(1–2):1–19.
Shah AC, Mlodozeniec AAR. Mechanism of surface lubrication: influence of duration of lubricant-excipient mixing on processing characteristics of powders and properties of compressed tablets. J Pharm Sci. 1977;66(10):1377–82.
Leinonen UI, Jalonen HU, Vihervaara PA, Laine ESU. Physical and lubrication properties of magnesium stearate. J Pharm Sci. 1992;81(12):1194–8.
Wang Y, Osorio JG, Li T, Muzzio FJ. Controlled shear system and resonant acoustic mixing: effects on lubrication and flow properties of pharmaceutical blends. Powder Technol. 2017;322:332–9.
Blackwood D, Ketterhagen W, Kresevic J, Kushner J, Moriarty J, Mullarney MP. Quantifying and reducing powder shear sensitivity when manufacturing capsules with lubricants. Drug Dev Ind Pharm. 2018;44(8):1350–6.
Ketterhagen RR, Mullarney MP, Kresevic J, Blackwood D. Computational approaches to predict the effect of shear during processing of lubricated pharmaceutical blends. Powder Technol. 2018;335:427–39.
Zhou QT, Qu L, Gengenbach T, Larson I, Stewart PJ, Morton DA. Effect of surface coating with magnesium stearate via mechanical dry powder coating approach on the aerosol performance of micronized drug powders from dry powder inhalers. AAPS PharmSciTech. 2013;14(1):38–44.
Braido D. Characterization and modeling the dissolution performance of tablets focusing on powder processing effects. 2012.
Hernandez E, Pawar P, Keyvan G, Wang Y, Velez N, Callegari G, et al. Prediction of dissolution profiles by non-destructive near infrared spectroscopy in tablets subjected to different levels of strain. J Pharm Biomed Anal. 2016;117:568–76.
Kushner J. Incorporating Turbula mixers into a blending scale-up model for evaluating the effect of magnesium stearate on tablet tensile strength and bulk specific volume. Int J Pharm. 2012;429(1):1–11.
Kushner J, Moore F. Scale-up model describing the impact of lubrication on tablet tensile strength. Int J Pharm. 2010;399(1):19–30.
Kushner J, Schlack AH. Commercial scale validation of a process scale-up model for lubricant blending of pharmaceutical powders. Int J Pharm. 2014;475(1):147–55.
Vanarase AU, Alcala M, Rozo JIJ, Muzzio FJ, Romanach RJ. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65(21):5728–33.
Moghtadernejad S, Escotet-Espinoza MS, Oka S, Singh R, Liu Z, Román-Ospino A, et al. A training on: continuous manufacturing (direct compaction) of solid dose pharmaceutical products. J Pharm Innov. 2018;13(2):155–87.
Liu Z, Wang Y, Muzzio FJ, Callegari G, Drazer G. Capillary drop penetration method to characterize the liquid wetting of powders. Langmuir. 2017;33(1):56–65.
Fell JT, Newton AJM. Determination of tablet strength by the diametral-compression test. J Pharm Sci. 1970;59(5):688–91.
Lantz B. The impact of sample non-normality on ANOVA and alternative methods. Br J Math Stat Psychol. 2013;66(2):224–44.
Khan A, Rayner GD. Robustness to non-normality of common tests for the many-sample location problem. J Appl Math Decis Sci. 2003;7(4):187–206.
Kanji GK. Effect of non-normality on the power in analysis of variance: a simulation study. Int J Math Educ Sci Technol. 1976;7(2):155–60.
Berthiaux H, Marikh K, Gatumel C. Continuous mixing of powder mixtures with pharmaceutical process constraints. Chem Eng Process. 2008;47(12):2315–22.
Marikh K, Berthiaux H, Barantseva MVE, Ponomarev D. Flow analysis and Markov chain modelling to quantify the agitation effect in a continuous powder mixer. Chem Eng Res Des. 2006;84(11):1059–74.
Van Snick B, Holman J, Cunningham C, Kumar A, Vercruysse J, De Beer T, et al. Continuous direct compression as manufacturing platform for sustained release tablets. Int J Pharm. 2017;519(1):390–407.
Acknowledgments
The authors would like to thank Mr. Lan Le, Mr. Kian Chau, Ms. Rhea Jamsandekar, and Ms. Tulsi Char for their support during the experiments. The authors also want to thank Dr. James Scicolone, Dr. Sonia Razavi and, Dr. Sarang Oka for their valuable inputs.
Equation parameters
-
MAcc mass hold up inside of the blender [=] g
-
\( {\dot{m}}_{in} \) mass flow rate into the blender [=] g/s
-
\( {\dot{m}}_{out} \) mass flow rate out of the blender [=] g/s
-
τspace − time space time in the blender [=] s
-
Nblade − passes number of blade passes experienced by the blend inside the blender
-
ω angular speed of blades in the blender [=] RPM
-
Kbatch extent of lubrication in a batch blending system [=] dm
-
δ geometric constant for extent of lubrication in batch systems [=] unitless
-
Vb batch blender volume [=] L
-
Fh fraction of headspace empty [=] unitless
-
Nrev number of revolutions of the blende [=] unitless
-
Kcontinuous extent of lubrication in continuous blending systems [=] dm
-
α geometric constant for extent of lubrication in continuous systems [=] unitless
-
vtip tip speed of blades in the continuous blending system [=] m/s
-
Dblender dimeter of the continuous blending system [=] m
-
Ksystem extent of lubrication observed by the blend [=] dm
-
σtablet, 0 tablet tensile strength at point of no lubrication [=] MPa
-
σtablet tablet tensile strength [=] MPa
-
γ lubrication extent coefficient of a blend [=] unitless
-
μ tensile strength decay rate of a blend [=] 1/dm
-
F tablet breaking force [=] N or kp
-
hT measured tablet thickness [=] mm
-
Dtablet tablet in-die diameter [=] mm
Funding
The research was financially supported by Janssen Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Moghtadernejad and M. Sebastian Escotet-Espinoza share equal first authorship.
Rights and permissions
About this article
Cite this article
Moghtadernejad, S., Escotet-Espinoza, M.S., Liu, Z. et al. Mixing Cell: a Device to Mimic Extent of Lubrication and Shear in Continuous Tubular Blenders. AAPS PharmSciTech 20, 262 (2019). https://doi.org/10.1208/s12249-019-1473-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1473-1