Abstract
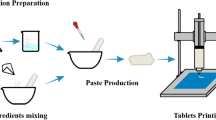
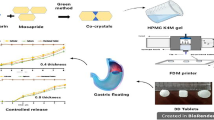
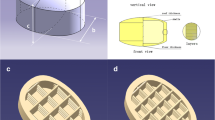
The objective of this study was to investigate the development of a novel puerarin gastric floating system with a concentric annular internal pattern by a 3D extrusion-based printing technique and to explore the flexibility of turning the release behavior through the design of the internal structure. The composition consisted of the conventional sustained-release pharmaceutical excipients without addition of foaming agent or light materials. First, the proper alcohol/water proportion was selected for the binding agent. The desired drug release behaviors and good floating properties were obtained either through modification of the formulation composition or adjustment of the internal structure. In vitro, the printed tablets were evaluated for drug release, mechanical properties, lag time, and floating duration time. The in vivo behaviors of the formulations were noted at certain time intervals through assessment of the radiographic pictures of healthy volunteers. The gastric retention time in the 3D-printed tablet was approximately 6 h in vivo. Results indicated these puerarin gastric floating 3D-printed tablets had great potential to achieve good gastric residence time and controlled release. Therefore, 3D extrusion-based printing appears to be appropriate for the production of oral administration systems, owing to its flexibility and the great floating ability and controlled-release capacity of its products.







Similar content being viewed by others
References
Awasthi R, Kulkarni GT. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand? Drug Delivery. 2014;23(2):378–94.
Porwal A, Dwivedi H, Pathak K, Porwal A, Dwivedi H, Pathak K. Decades of research in drug targeting using gastroretentive drug delivery systems for antihypertensive therapy. Brazjpharmsci. 2017;53(3).
Sathish D, ., Himabindu S, ., Y Shravan K, Y Madhusudan R. Floating drug delivery systems for prolonging gastric residence time: a review. Current Drug Delivery 2011;8(5):-, 494, 510.
Yu DG, Zhu LM, Branford-White CJ, Yang XL. Three-dimensional printing in pharmaceutics: promises and problems. J Pharm Sci. 2008;97(9):3666–90.
D’Aveni R. The 3-D printing revolution. ICIS Chem Bus. 2015.
Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Technol. 2015;30:360–7.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. European Journal of Pharmaceutical Sciences Official Journal of the European Federation for Pharmaceutical Sciences. 2015;68:11–7.
Pietrzak K, Isreb A, Alhnan MA. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. 2015;96:380–7.
Alvaro G, Pamela RM, Asma B, Basit AW, Simon G. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63.
Yu D, Branford-White C, Zh ZL, Li X, Yang X. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int J Pharm. 2009;370(1):160–6.
Sadia M, Arafat B, Ahmed W, Forbes RE, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2017;269:1–32.
Li Q, Guan X, Cui M, Zhu Z, Chen K, Wen H, et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int J Pharm. 2018;535(1–2):325–32.
Goyanes A, Buanz AB, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476(1–2):88–92.
On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur J Pharm Biopharm. 2018;134:29–36.
Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261:207–15.
Leong KF, Chua CK, Gui WS, Verani. Building porous biopolymeric microstructures for controlled drug delivery devices using selective laser sintering. Int J Adv Manuf Technol 2006;31(5–6):483–489.
Martinez PR, Goyanes A, Basit AW, Gaisford S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech. 2018:1–7.
Ji F, Zhang C, Chen X, editors. Structure optimization of porous dental implant based on 3D printing. Materials science and engineering conference series; 2018, 324, 012060.
Seng HL, Chia SMY, Kang L, Yap YL. Three-dimensional printing of carbamazepine sustained-release scaffold. J Pharm Sci. 2016;105(7):2155–63.
Tagami T, Nagata N, Hayashi N, Ogawa E, Fukushige K, Sakai N, et al. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int J Pharm. 2018;543:361–7.
Okwuosa TC, Stefaniak D, Arafat B, Isreb A, Wan KW, Alhnan MA. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm Res. 2016;33(11):2704–12.
Kollamaram G, Croker DM, Walker GM, Goyanes A, Basit AW, Gaisford S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int J Pharm. 2018;545(1–2):144–52.
Khaled SA, Burley JC, Alexander MR, Jing Y, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–50.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Khaled SA, Alexander MR, Wildman RD, Wallace MJ, Sharpe S, Yoo J, et al. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm. 2018;538(1–2):223–30.
Khirwadkar P, Dashora K. A review: fast dissolving drug delivery system: current developments in novel system design and technology. International Journal of Biomedical & Advance Research. 2012;3(2).
Rattanakit P, Moulton SE, Santiago KS, Liawruangrath S, Wallace GG. Extrusion printed polymer structures: a facile and versatile approach to tailored drug delivery platforms. Int J Pharm. 2012;422(1):254–63.
Choi YM, Jun HJ, Dawson K, Rodriguez RL, Mi RR, Jun J, et al. Effects of the isoflavone puerarin and its glycosides on melanogenesis in B16 melanocytes. Eur Food Res Technol. 2010;231(1):75–83.
Luo CF, Yuan M, Chen MS, Liu SM, Zhu L, Huang BY, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410(1):138–44.
Zhang Y, Wang R, Wu J, Shen Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int J Pharm. 2012;427(2):337–44.
Shengmiao C, Chunshun Z, Xing T, Dawei C, Zhonggui H. Study on the bioavailability of puerarin from Pueraria lobata isoflavone self-microemulsifying drug-delivery systems and tablets in rabbits by liquid chromatography-mass spectrometry. Biomed Chromatogr. 2010;19(5):375–8.
Yan-Hong WU, Zi-Ren SU, Lai XP, Lin J. Pharmacokinetics and bioavailability of Yufeng Ningxin tablet in beagle dog. Chinese Traditional Patent Medicine. 2006;28(2):215–8.
Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. Journal of Controlled Release Official Journal of the Controlled Release Society. 2000;63(3):235–59.
Goyanes A, Buanz ABM, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. European Journal of Pharmaceutics & Biopharmaceutics Official Journal of Arbeitsgemeinschaft Für Pharmazeutische Verfahrenstechnik E V 2015;89:157–162.
Darade A, Pathak S, Sharma S, Patravale V. Atovaquone oral bioavailability enhancement using electrospraying technology. European Journal of Pharmaceutical Sciences Official Journal of the European Federation for Pharmaceutical Sciences. 2017;111:195.
Shah VP, Tsong Y, Sathe P, Liu J-P. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15(6):889–96.
Zhu LX, Zhi C, Ying G, Huang SW, Shi SL, Hui L, et al. Correlation of in vitro release between total flavonoids and different ingredients in sustained-release pellets of Ginkgo biloba extract by f_2 fit factor method. Chinese traditional & herbal drugs. 2015.
Karalis V, Magklara E, Shah VP, Macheras P. From drug delivery systems to drug release, dissolution, IVIVC, BCS, BDDCS, bioequivalence and biowaivers. Pharm Res. 2010;27(9):2018–29.
Meka VS, Songa AS, Nali SR, Battu JR, Kolapalli VR. Design and in vitro evaluation of effervescent gastric floating drug delivery systems of propanolol HCl. Investig Clin. 2012;53(1):60–70.
Zhang X, Zhang Y, Han H, Yang J, Xu B, Wang B, et al. Formulation optimization of gastro-retention tablets of paeonol and efficacy in treatment of experimental gastric ulcer. Chem Pharm Bull. 2017;65(8):706–13.
Arza RA, Gonugunta CS, Veerareddy PR. Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets. AAPS PharmSciTech. 2009;10(1):220–6.
Kumar N, Soni S, Singh T, Kumar A, Ahmad FJ, Bhatnagar A, et al. Development and optimization of gastro-retentive controlled-release tablet of calcium-disodium edentate and its in vivo gamma scintigraphic evaluation. AAPS PharmSciTech. 2015;16(6):1270–80.
Sharma BG, Khanna K, Kumar N, Nishad DK, Basu M, Bhatnagar A. Development and gamma scintigraphy evaluation of gastro retentive calcium ion-based oral formulation; an innovative approach for the management of gastro-oesophageal reflux disease (GERD). Drug Dev Ind Pharm. 2017;1.
Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461(1–2):105–11.
Samana H, Grandy DB, Mike R, Craig DQM. A study of phase separation in peptide-loaded HPMC films using T(zero)-modulated temperature DSC, atomic force microscopy, and scanning electron microscopy. J Pharm Sci. 2004;93(7).
Reynhardt EC. An NMR, DSC and X-ray investigation of the disaccharides sucrose, maltose and lactose. Mol Phys. 1990;69(6):1083–97.
Craig DQM, Barsnes M, Royall PG, Kett VL. An evaluation of the use of modulated temperature DSC as a means of assessing the relaxation behaviour of amorphous lactose. Pharm Res. 2000;17(6):696–700.
Lerk CF, Andreae AC, Boer AHD, Hoog PD, Kussendrager K, Leverink JV. Alterations of α-lactose during differential scanning calorimetry. J Pharm Sci. 1984;73(6):856–7.
Hussain S, Grandy DB, Reading M, Craig DQM. A study of phase separation in peptide-loaded HPMC films using T zero -modulated temperature DSC, atomic force microscopy, and scanning electron microscopy. J Pharm Sci. 2004;93(7):1672–81.
Acknowledgments
We are grateful for the instrumental support received from JingXin Pharmaceutical Co., Ltd. (Zhejiang, China).
Funding
This work was supported by the National Science and Technology Major Project, belonging to the “Research on the key technology of new drug delivery system and industrialization of new projects” line (no. 2014ZX09507001004), and by the Open fund of the Key Laboratory of the Ministry of Education for TCM Viscera-State Theory and Applications, Liaoning University of Traditional Chinese Medicine, belonging to the “Research of gastro-retentive and control-released preparation of ginkgolide” line (no. zyzx1608).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving human participants in this study conformed to the ethical standards of the institutional and/or national research committee and complied with the 1964 Helsinki declaration and its later amendments and with other comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, P., Zhang, S., Sun, W. et al. Flexibility of 3D Extruded Printing for a Novel Controlled-Release Puerarin Gastric Floating Tablet: Design of Internal Structure. AAPS PharmSciTech 20, 236 (2019). https://doi.org/10.1208/s12249-019-1455-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1455-3