Abstract
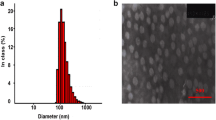
Mitoxantrone (MTO) is used to treat certain types of cancer, mostly metastatic cancer. While the drug has poor aqueous solubility and high side effects. Self-assembly nanocrystal is a novel lymphatic targeting delivery system. In our study, MTO self-assembly nanocrystal (MTO NC) was successfully prepared to improve lymphatic targeting ability and reduce its toxicity. MTO NCs had small size, stable potential, and uniform distribution. The average particle size of MTO NCs was less than 100 nm with the 0.218 PDI and − 6.6 mV the Zeta potential value. TEM images showed that MTO NCs had a sphere-like morphology with smooth surface and uniform distribution; Atomic force microscopy (AFM) images gave a 3D surface of MTO NCs. Polarizing microscope micrograph (PLM) of MTO NCs in lymph nodes demonstrated the crystal structure of MTO NCs when it was exposed to physiological condition. Transmission electron microscopy showed the presence of MTO NCs in mice lymph nodes. Pharmacokinetic parameters of MTO strongly demonstrated that MTO NCs could target the lymph nodes after subcutaneous injection. Moreover, tissue distribution results indicated that MTO NCs were mainly absorbed by the lymphatics and reduced system toxicity. Finally, a lymphatic metastasis mice model was established to precede the pharmacodynamics of MTO NCs, and using MTO liposomes as a reference preparation, the inhibitory effect of MTO NCs on lymphatic metastasis was markedly higher. Briefly, MTO NCs, as a novel self-assembled lymphatic targeting system, can accumulate in the metastatic lymph nodes and lead anticancer drug to kill cancer cells and control lymphatic metastasis with extremely low systemic toxicity.









Similar content being viewed by others
References
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33.
Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71:1214–8.
van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for research and treatment of cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–50.
Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189(5):2181–90.
Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1(3):191–9.
Betticher DC, Hsu Schmitz S-F, Tötsch M, Hansen E, Joss C, von Briel C, et al. Mediastinal lymph node clearance After docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non–small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21:1752–9.
Alvarado R, Yi M, Le-Petross H, Gilcrease M, Mittendorf EA, Bedrosian I, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177–84.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92.
Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. 2014;124:874–7.
Mohrman DE, Heller LJ, Physiology CV. 6th. New York: McGraw-Hill Companies; 2006.
Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–77.
Pawar VK, Singh Y, Meher JG. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66.
Zhang XY, Lu WY. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer Biol Med. 2014;11(4):247–54.
Lehn JM. Toward self-organization and complex matter. Science. 2002;295:2400–3.
Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8.
Mendes AC, Baran ET, Reis RL, Azevedo HS. Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wires Nanomed Nanobiol. 2013;5:582–612.
Sosnik A. Drug self-assembly: a phenomenon at the nanometer scale with major impact in the structure–biological properties relationship and the treatment of disease. Prog Mater Sci. 2016;82:39–82.
Lock LL, La Comb M, Schwarz K, Cheetham AG, Lin Y, Zhang P. Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Faraday Discuss. 2013;166:285–301.
Taboada P, Gutierrez-Pichel M, Mosquera V. Chem Phys. 2004;298:65–74.
Coltman CA Jr, McDaniel TM, Balcerzak SP. Mitoxantrone hydrochloride (NSC-310739) in lymphoma. Invest New Drug. 1983;1(1):65–70.
Beijnen JH, Bult A, Underberg WJM, Analytical profiles of drug substances. 1988;17:221–258
Supersaxo A, Hein WR, Steffen H. Pharm Res. 1990;7(2):167–9.
Porter CJ, Charman WN. Adv Drug Deliv Rew. 2001;50(1–2):1.
Liu J,et al. Bio-self-assembled nanocrystalline injection with lymphatic targeting function and its preparation method. 2017;CN201710486510.8.
Li J, King AV, Stickel SL. Vaccine. 2007;27(4):558–64.
Ugwu S, Zhang A, Parmar M. Preparation, characterization, and stability of liposome-based formulations of mitoxantrone. Drug Dev Ind Pharm. 2005;31(2):223–9.
Schwendener RA, Fiebig HH, Berger MR, Berger DP. Evaluation of incorporation characteristics of mitoxantrone into unilamellar liposomes and analysis of their pharmacokinetic properties, acute toxicity, and antitumor efficacy*. Cancer Chemother Pharmacol. 1991;27(6):429–39.
Wang J, Kang WM, Yu JC, Liu YQ, Meng QB, Cao ZJ. Cadherin-17 induces tumorigenesis and lymphatic metastasis in gastric cancer through activation of NFκB signaling pathway. Cancer Biol Ther. 2013;14:262–70.
Xiong SB, Lu B, Yang H, et al. Study on the tissue distribution and lymph node targeting of mitoxantrone loaded albumin nanoparticles RP-HPLC method for determination of mitoxantrone in rat plasma and different tissues. Chin J Pharm Anal. 2006;26(8):1043–9.
Rentsch K, Horber D, Schwendener R, Wunderli-Allenspach H, Hänseler E. Comparative pharmacokinetic and cytotoxic analysis of three different formulations of mitoxantrone in mice. Br J Cancer. 1997;75(7):986–92.
Law SL, Jang TF, Chang P, et al. Release characteristics of mitoxantrone-containing liposomes. Int J Pharm. 1994;103(1):81–5.
Sato DYO, Wal R, De Oliveira CC. Histopathological and immunophenotyping studies on normal and sarcoma 180-bearing mice treated with a complex homeopathic medication. Homeopathy. 2005;94(1):26–32.
Acknowledgments
We wish to acknowledge the support of Pharmacy Laboratory Centre and Animal Centre of Shenyang Pharmaceutical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, Y., Liu, J., Shi, T. et al. A Novel Self-Assembly Nanocrystal as Lymph Node-Targeting Delivery System: Higher Activity of Lymph Node Targeting and Longer Efficacy Against Lymphatic Metastasis. AAPS PharmSciTech 20, 292 (2019). https://doi.org/10.1208/s12249-019-1447-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1447-3