Abstract
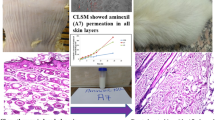
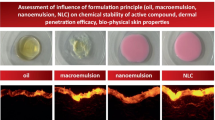
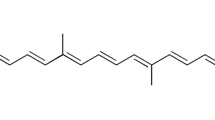
All-trans retinoic acid (ATRA) has been regarded as a wonder drug for many dermatological complications; however, its application is limited due to the extreme irritation, and toxicity seen once it has sufficiently concentrated into the bloodstream from the skin. Thus, the present study was aimed to increase the entrapment of ATRA and minimize its transdermal permeation. ATRA incorporated within nanostructured lipid carriers (NLCs) were produced by a green and facile thin lipid-film based microwave-assisted rapid technique (MART). The optimization was carried out using the response surface methodology (RSM)-driven artificial neural network (ANN) coupled with genetic algorithm (GA). The liquid lipid and surfactants were seen to play a very crucial role culminating in the particle size (< 70 nm), zeta potential (< − 32 mV), and entrapment of ATRA (> 98%). ANN-GA-optimized NLCs required a minimal quantity of the surfactants, formed within 2 min and were stable for 1 year at different storage conditions. The optimized NLC-loaded creams showed a skin retention (ex vivo) to an extent of 87.42% with no detectable drug in the receptor fluid (24 h) in comparison to the marketed cream which released 47.32% (12 h) of ATRA. The results were in good correlation with the in vivo skin deposition studies. The NLCs were biocompatible and non-skin irritant based on the primary irritation index. In conclusion, the NLCs were seen to have a very high potential in overcoming the drawbacks of ATRA for dermal delivery and could be produced conveniently by the MART.












Similar content being viewed by others
References
Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. https://doi.org/10.1016/j.biopha.2018.04.055.
Li Q, Cai T, Huang Y, Xia X, Cole SPC, Cai Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomater (Basel, Switzerland). 2017;7(6). https://doi.org/10.3390/nano7060122.
Courant T, Bayon E, Reynaud-Dougier HL, Villiers C, Menneteau M, Marche PN, et al. Tailoring nanostructured lipid carriers for the delivery of protein antigens: physicochemical properties versus immunogenicity studies. Biomaterials. 2017;136:29–42. https://doi.org/10.1016/j.biomaterials.2017.05.001.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–55. https://doi.org/10.1016/S0169-409X(02)00118-7.
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharmacy, Cairo Univ. 2015;53(2):147–59. https://doi.org/10.1016/j.bfopcu.2015.10.001.
Beg S, Saini S, Bandopadhyay S, Katare OP, Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm. 2018;44(3):407–20. https://doi.org/10.1080/03639045.2017.1395459.
Chanburee S, Tiyaboonchai W. Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Dev Ind Pharm. 2017;43(3):432–40. https://doi.org/10.1080/03639045.2016.1257020.
Kasongo KW, Müller RH, Walker RB. The use of hot and cold high pressure homogenization to enhance the loading capacity and encapsulation efficiency of nanostructured lipid carriers for the hydrophilic antiretroviral drug, didanosine for potential administration to paediatric patients. Pharm Dev Technol. 2012;17(3):353–62. https://doi.org/10.3109/10837450.2010.542163.
Bhagurkar AM, Repka MA, Murthy SN. A novel approach for the development of a nanostructured lipid carrier formulation by hot-melt extrusion technology. J Pharm Sci. 2017;106(4):1085–91. https://doi.org/10.1016/J.XPHS.2016.12.015.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. https://doi.org/10.1208/s12249-015-0360-7.
Shah RM, Bryant G, Taylor M, Eldridge DS, Palombo EA, Harding IH. Structure of solid lipid nanoparticles produced by a microwave-assisted microemulsion technique. RSC Adv. 2016;6(43):36803–10. https://doi.org/10.1039/C6RA02020H.
Cavalcanti SMT, Nunes C, Costa Lima SA, Soares-Sobrinho JL, Reis S. Optimization of nanostructured lipid carriers for zidovudine delivery using a microwave-assisted production method. Eur J Pharm Sci. 2018;122:22–30. https://doi.org/10.1016/J.EJPS.2018.06.017.
Zhang H. Thin-film hydration followed by extrusion method for liposome preparation. In: Methods in molecular biology (Clifton, NJ); 2017. p. 17–22. https://doi.org/10.1007/978-1-4939-6591-5_2.
Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258(1–2):141–51. https://doi.org/10.1016/S0378-5173(03)00183-2.
Vakilinezhad MA, Tanha S, Montaseri H, Dinarvand R, Azadi A, Akbari Javar H. Application of response surface method for preparation, optimization, and characterization of nicotinamide loaded solid lipid nanoparticles. Adv Pharm Bull. 2018;8(2):245–56. https://doi.org/10.15171/apb.2018.029.
Samson S, Basri M, Fard Masoumi HR, Abdul Malek E, Abedi Karjiban R. An artificial neural network based analysis of factors controlling particle size in a virgin coconut oil-based nanoemulsion system containing copper peptide. Mukherjee A, editor. PLoS One. 2016;11(7):e0157737. https://doi.org/10.1371/journal.pone.0157737.
Ghaheri A, Shoar S, Naderan M, Hoseini SS. The applications of genetic algorithms in medicine. Oman Med J. 2015;30(6):406–16. https://doi.org/10.5001/omj.2015.82.
Sha W, Edwards KL. The use of artificial neural networks in materials science based research. Mater Des. 2007;28(6):1747–52. https://doi.org/10.1016/J.MATDES.2007.02.009.
Leyden J, Stein-Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther (Heidelb). 2017;7(3):293–304. https://doi.org/10.1007/s13555-017-0185-2.
Ghate VM, Lewis SA, Prabhu P, Dubey A, Patel N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur J Pharm Biopharm. 2016;108:253–61. https://doi.org/10.1016/j.ejpb.2016.07.026.
Yamaguchi Y, Nagasawa T, Nakamura N, Takenaga M, Mizoguchi M, Kawai S, et al. Successful treatment of photo-damaged skin of nano-scale atRA particles using a novel transdermal delivery. J Control Release. 2005;104(1):29–40. https://doi.org/10.1016/j.jconrel.2004.11.036.
Castleberry SA, Quadir MA, Sharkh MA, Shopsowitz KE, Hammond PT. Polymer conjugated retinoids for controlled transdermal delivery. J Control Release. 2017;262:1–9. https://doi.org/10.1016/j.jconrel.2017.07.003.
Shah K, Date A, Joshi M, Patravale V. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345(1–2):163–71. https://doi.org/10.1016/j.ijpharm.2007.05.061.
Lapteva M, Möller M, Gurny R, Kalia YN. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale. 2015;7(44):18651–62. https://doi.org/10.1039/C5NR04770F.
Kircik LH. Microsphere technology: hype or help? J Clin Aesthet Dermatol. 2011;4(5):27–31.
Raza K, Singh B, Lohan S, Sharma G, Negi P, Yachha Y, et al. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int J Pharm. 2013;456(1):65–72. https://doi.org/10.1016/j.ijpharm.2013.08.019.
Sureka S, Gupta G, Agarwal M, Mishra A, K. Singh S, P. Singh R, et al. Formulation, in-vitro and ex-vivo evaluation of tretinoin loaded cubosomal gel for the treatment of acne. Recent Pat Drug Deliv Formul 2018;12(2):121–129. https://doi.org/10.2174/1872211312666180213121117.
Rahman SA, Abdelmalak NS, Badawi A, Elbayoumy T, Sabry N, Ramly A El. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: in-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv 2015;22(6):731–739. https://doi.org/10.3109/10717544.2014.896428.
Shetty PK, Venuvanka V, Jagani HV, Chethan GH, Ligade VS, Musmade PB, et al. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int J Nanomedicine. 2015;10:6477–91. https://doi.org/10.2147/IJN.S90964.
Shah NV, Seth AK, Balaraman R, Aundhia CJ, Maheshwari RA, Parmar GR. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: design and in vivo study. J Adv Res. 2016;7(3):423–34. https://doi.org/10.1016/j.jare.2016.03.002.
Monteiro LM, Löbenberg R, Cotrim PC, Barros de Araujo GL, Bou-Chacra N. Buparvaquone nanostructured lipid carrier: development of an affordable delivery system for the treatment of leishmaniases. Biomed Res Int. 2017;2017:1–11. https://doi.org/10.1155/2017/9781603.
Dhanarajan G, Rangarajan V, Bandi C, Dixit A, Das S, Ale K, et al. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J Biotechnol. 2017;256:46–56. https://doi.org/10.1016/j.jbiotec.2017.05.007.
Baskar G, Renganathan S. Optimization of L-asparaginase production by Aspergillus terreus MTCC 1782 using response surface methodology and artificial neural network-linked genetic algorithm. Asia-Pacific J Chem Eng. 2012;7(2):212–20. https://doi.org/10.1002/apj.520.
Kodoth AK, Ghate VM, Lewis SA, Badalamoole V. Application of pectin-zinc oxide hybrid nanocomposite in the delivery of a hydrophilic drug and a study of its isotherm, kinetics and release mechanism. Int J Biol Macromol. 2018;115:418–30. https://doi.org/10.1016/j.ijbiomac.2018.04.069.
Guo T, Zhang Y, Zhao J, Zhu C, Feng N. Nanostructured lipid carriers for percutaneous administration of alkaloids isolated from Aconitum sinomontanum. J Nanobiotechnol. 2015;13:47. https://doi.org/10.1186/s12951-015-0107-3.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71. https://doi.org/10.1208/s12248-010-9185-1.
Alam S, Aslam M, Khan A, Imam SS, Aqil M, Sultana Y, et al. Nanostructured lipid carriers of pioglitazone for transdermal application: from experimental design to bioactivity detail. Drug Deliv. 2016;23(2):601–9. https://doi.org/10.3109/10717544.2014.923958.
Zhao J, Piao X, Shi X, Si A, Zhang Y, Feng N. Podophyllotoxin-loaded nanostructured lipid carriers for skin targeting: in vitro and in vivo studies. Molecules. 2016;21(11):1549. https://doi.org/10.3390/molecules21111549.
Chen Y, Zhou L, Yuan L, Zhang Z, Liu X, Wu Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int J Nanomedicine. 2012;7:3023–32. https://doi.org/10.2147/IJN.S32476.
Banerjee S, Chattopadhyay P, Ghosh A, Pathak MP, Singh S, Veer V. Acute dermal irritation, sensitization, and acute toxicity studies of a transdermal patch for prophylaxis against (±) anatoxin-A poisoning. Int J Toxicol. 2013;32(4):308–13. https://doi.org/10.1177/1091581813489996.
Wang J, Li Z, Sun F, Tang S, Zhang S, Lv P, et al. Evaluation of dermal irritation and skin sensitization due to vitacoxib. Toxicol Rep. 2017;4:287–90. https://doi.org/10.1016/j.toxrep.2017.06.003.
Simonsen L, Petersen MB, Groth L. In vivo skin penetration of salicylic compounds in hairless rats. Eur J Pharm Sci. 2002;17(1–2):95–104. https://doi.org/10.1016/S0928-0987(02)00147-1.
Mohammed D, Matts PJ, Hadgraft J, Lane ME. In vitro–in vivo correlation in skin permeation. Pharm Res. 2014;31(2):394–400. https://doi.org/10.1007/s11095-013-1169-2.
Sinico C, Manconi M, Peppi M, Lai F, Valenti D, Fadda AM. Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle–skin interaction. J Control Release. 2005;103(1):123–36. https://doi.org/10.1016/J.JCONREL.2004.11.020.
Li Y, Abbaspour MR, Grootendorst PV, Rauth AM, Wu XY. Optimization of controlled release nanoparticle formulation of verapamil hydrochloride using artificial neural networks with genetic algorithm and response surface methodology. Eur J Pharm Biopharm. 2015;94:170–9. https://doi.org/10.1016/J.EJPB.2015.04.028.
Metwally AA, Hathout RM. Computer-assisted drug formulation design: novel approach in drug delivery. Mol Pharm. 2015;12(8):2800–10. https://doi.org/10.1021/mp500740d.
Gao Y, Chang M-W, Ahmad Z, Li J-S. Magnetic-responsive microparticles with customized porosity for drug delivery. RSC Adv. 2016;6(91):88157–67. https://doi.org/10.1039/C6RA17162A.
Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, et al. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016;8:163–76. https://doi.org/10.2147/CPAA.S64788.
Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–48.
Veraldi S, Barbareschi M, Benardon S, Schianchi R. Short contact therapy of acne with tretinoin. J Dermatol Treat. 2013;24(5):374–6. https://doi.org/10.3109/09546634.2012.751085.
Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9. https://doi.org/10.1034/j.1600-0625.2000.009003165.x.
Bouwstra JA. The skin barrier, a well-organized membrane. Colloids Surfaces A Physicochem Eng Asp. 1997;123–124:403–13. https://doi.org/10.1016/S0927-7757(96)03819-8.
Hafeez F, Maibach H. Occlusion effect on in vivo percutaneous penetration of chemicals in man and monkey: partition coefficient effects. Skin Pharmacol Physiol. 2013;26(2):85–91. https://doi.org/10.1159/000346273.
Garg A, Singh S. Targeting of eugenol-loaded solid lipid nanoparticles to the epidermal layer of human skin. Nanomedicine (Lond). 2014;9(8):1223–38. https://doi.org/10.2217/nnm.13.33.
Santos Maia C, Mehnert W, Schaller M, Korting HC, Gysler A, Haberland A, et al. Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target. 2002;10(6):489–95. https://doi.org/10.1080/1061186021000038364.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168(2):221–9. https://doi.org/10.1016/S0378-5173(98)00092-1.
Acknowledgements
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, for providing financial assistance in the form of Senior Research Fellowship (SRF) to Vivek M. Ghate [File No. 8/602(0003)/18 EMR-1]. The authors are also thankful to Mangalore University, Mangalagangotri, for their support in analyzing the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 8406 kb)
Rights and permissions
About this article
Cite this article
Ghate, V.M., Kodoth, A.K., Raja, S. et al. Development of MART for the Rapid Production of Nanostructured Lipid Carriers Loaded with All-Trans Retinoic Acid for Dermal Delivery. AAPS PharmSciTech 20, 162 (2019). https://doi.org/10.1208/s12249-019-1307-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1307-1