Abstract
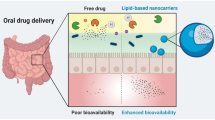
Oral drug delivery route is one of the most convenient and extensively utilised routes for drug administration. But there exists class of drugs which exhibit poor bioavailability on oral drug administration. Designing of drug–lipid conjugates (DLCs) is one of the rationale strategy utilised in overcoming this challenge. This review extensively covers the various dimensions of drug modification using lipids to attain improved oral drug delivery. DLCs help in improving oral delivery by providing benefits like improved permeability, stability in gastric environment, higher drug loading in carriers, formation of self-assembled nanostructures, etc. The clinical effectiveness of DLCs is highlighted from available marketed drug products along with many DLCs in phase of clinical trials. Conclusively, this drug modification strategy can potentially help in augmenting oral drug delivery in future.




Similar content being viewed by others
References
Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery—a review. Pharm Sci Technolol Today. 2000;3:138–45.
Charman WN. Lipids, lipophilic drugs, and oral drug delivery—some emerging concepts. J Pharm Sci. 2000;89:967–78.
Taylor MD. Improved passive oral drug delivery via prodrugs. Adv Drug Deliv Rev. 1996;19:131–48.
Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. Prodrug approach: an overview of recent cases. Eur J Med Chem. Elsevier Masson. 2017;127:810–27.
Wermuth CG. Designing prodrugs and bioprecursors. In: Pract Med Chem. 3rd ed. Cambridge: Academic Press; 2008. p. 721–46.
Kokil GR, Rewatkar PV. Bioprecursor prodrugs: molecular modification of the active principle. Mini-Reviews Med Chem. 2010;10:1316–30.
Silverman RB, Holladay MW. The organic chemistry of drug design and drug action. 3rd ed. Drug Dev. Res. The Academic Press; 2014.
Lambert DM. Rationale and applications of lipids as prodrug carriers. Eur J Pharm Sci. Elsevier. 2000;11:S15–27.
Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci Elsevier. 2006;95:1177–95.
Huttunen KM, Raunio H, Rautio J. Prodrugs—from serendipity to rational design. Pharmacol Rev. 2011;63:750–71.
Clas S-D, Sanchez RI, Nofsinger R. Chemistry-enabled drug delivery (prodrugs): recent progress and challenges. Drug Discov Today. Elsevier Current Trends. 2014;19:79–87.
Lesniewska-Kowiel MA, Muszalska I. Strategies in the designing of prodrugs, taking into account the antiviral and anticancer compounds. Eur J Med Chem Elsevier Masson. 2017;129:53–71.
Müller RH, Olbrich C. Lipid matrix-drug conjugates particle for controlled release of active ingredient [Internet]. 2000 [cited 2018 Nov 22]. Available from: patents.google.com/patent/US6770299B1/en. Accessed 19 Sept 2018.
Adhikari P, Pal P, Das AK, Ray S, Bhattacharjee A, Mazumder B. Nano lipid-drug conjugate: an integrated review. Int J Pharm. 2017;529:629–41.
Kondo S, Hosaka S, Hatakeyama I, Kuzuya M. Mechanochemical solid-state polymerization. IX. Theoretical analysis of rate of drug release from powdered polymeric prodrugs in a heterogeneous system. Chem Pharm Bull (Tokyo). The Pharmaceutical Society of Japan. 1998;46:1918–23.
D’Souza AJM, Topp EM. Release from polymeric prodrugs: linkages and their degradation. J Pharm Sci. 2004;93:1962–79.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 67:217–23.
Siepmann J. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev Elsevier. 2012;64:163–74.
Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-matrices for controlled drug delivery: a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res. Kluwer Academic Publishers-Plenum Publishers. 1999;16:1748–56.
Pitt GG, Cha Y, Shah SS, Zhu KJ. Blends of PVA and PGLA: control of the permeability and degradability of hydrogels by blending. J Control Release. Elsevier. 1992;19:189–99.
Irby D, Du C, Li F. Lipid–drug conjugate for enhancing drug delivery. Mol Pharm. American Chemical Society. 2017;14:1325–38.
Teshima M, Fumoto S, Nishida K, Nakamura J, Ohyama K, Nakamura T, et al. Prolonged blood concentration of prednisolone after intravenous injection of liposomal palmitoyl prednisolone. J Control Release. Elsevier. 2006;112:320–8.
Signorell RD, Luciani P, Brambilla D, Leroux J-C. Pharmacokinetics of lipid-drug conjugates loaded into liposomes. Eur J Pharm Biopharm. Elsevier. 2018;128:188–99.
Zalipsky S, Gabizon AA. Conjugate having a cleavable linkage for use in a liposome [Internet]. 2000 [cited 2018 Nov 22]. Available from: https://patents.google.com/patent/US6365179B1/en. Accessed 19 Sept 2018.
McDonald GB, Weidman M. Partitioning of polar fatty acids into lymph and portal vein after intestinal absorption in the rat. Q J Exp Physiol. Wiley/Blackwell (10.1111). 1987;72:153–9.
Alexander P, Kucera G, Pardee TS. Improving nucleoside analogs via lipid conjugation: is fatter any better? Crit Rev Oncol Hematol. Elsevier. 2016;100:46–56.
Wang Y, Li L, Jiang W, Larrick JW. Synthesis and evaluation of a DHA and 10-hydroxycamptothecin conjugate. Bioorg Med Chem Pergamon. 2005;13:5592–9.
Dichwalkar T, Patel S, Bapat S, Pancholi P, Jasani N, Desai B, et al. Omega-3 fatty acid grafted PAMAM-paclitaxel conjugate exhibits enhanced anticancer activity in upper gastrointestinal cancer cells. Macromol Biosci. Wiley-Blackwell. 2017;17:1600457.
Bedikian AY, DeConti RC, Conry R, Agarwala S, Papadopoulos N, Kim KB, et al. Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol Oxford University Press. 2011;22:787–93.
Venugopal B, Awada A, Evans TRJ, Dueland S, Hendlisz A, Rasch W, et al. A first-in-human phase I and pharmacokinetic study of CP-4126 (CO-101), a nucleoside analogue, in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2015;76:785–92.
Pardini RS. Nutritional intervention with omega-3 fatty acids enhances tumor response to anti-neoplastic agents. Chem Biol Interact Elsevier. 2006;162:89–105.
Effenberger K, Breyer S, Schobert R. Modulation of doxorubicin activity in cancer cells by conjugation with fatty acyl and terpenyl hydrazones. Eur J Med Chem. Elsevier Masson. 2010;45:1947–54.
Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett Elsevier. 2000;148:173–9.
Sun B, Luo C, Cui W, Sun J, He Z. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J Control Release. Elsevier. 2017;264:145–59.
Bontemps L, Demaison L, Keriel C, Pernin C, Mathieu JP, Marti-Batlle D, et al. Kinetics of (16 123I) Iodohexadecenoic acid metabolism in the rat myocardium, influence of glucose concentration in the perfusate and comparison with (1 14C) palmitate. Eur Heart J Oxford University Press. 1985;6:91–6.
Charbon V, Latour I, Lambert DM, Buc-Calderon P, Neuvens L, De Keyser J, et al. Targeting of drug to the hepatocytes by fatty acids. Influence of the carrier (albumin or galactosylated albumin) on the fate of the fatty acids and their analogs. Pharm Res. Kluwer Academic Publishers-Plenum Publishers. 1996;13:27–31.
Sparreboom A, Verweij J, van der Burg ME, Loos WJ, Brouwer E, Viganò L, et al. Disposition of Cremophor EL in humans limits the potential for modulation of the multidrug resistance phenotype in vivo. Clin Cancer Res American Association for Cancer Research. 1998;4:1937–42.
Stuurman FE, Voest EE, Awada A, Witteveen PO, Bergeland T, Hals P-A, et al. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Invest New Drugs Springer US. 2013;31:959–66.
Pignata S, Amant F, Scambia G, Sorio R, Breda E, Rasch W, et al. A phase I-II study of elacytarabine (CP-4055) in the treatment of patients with ovarian cancer resistant or refractory to platinum therapy. Cancer Chemother Pharmacol Springer-Verlag. 2011;68:1347–53.
Bala V, Rao S, Bateman E, Keefe D, Wang S, Prestidge CA. Enabling oral SN38-based chemotherapy with a combined lipophilic prodrug and self-microemulsifying drug delivery system. Mol Pharm American Chemical Society. 2016;13:3518–25.
Liu J, Liu J, Zhao D, Ma N, Luan Y. Highly enhanced leukemia therapy and oral bioavailability from a novel amphiphilic prodrug of cytarabine. RSC Adv. The Royal Society of Chemistry. 2016;6:35991–9.
Kandula M, Sunil Kumar K, Palanichamy S, Rampal A. Discovery and preclinical development of a novel prodrug conjugate of mesalamine with eicosapentaenoic acid and caprylic acid for the treatment of inflammatory bowel diseases. Int Immunopharmacol Elsevier. 2016;40:443–51.
Han S, Hu L, Quach T, Simpson JS, Trevaskis NL, Porter CJH. Profiling the role of deacylation-reacylation in the lymphatic transport of a triglyceride-mimetic prodrug. Pharm Res Springer US. 2015;32:1830–44.
Han S, Hu L, Gracia, Quach T, Simpson JS, Edwards GA, et al. Lymphatic transport and lymphocyte targeting of a triglyceride mimetic prodrug is enhanced in a large animal model: studies in greyhound dogs. Mol pharm. Am Chem Soc. 2016;13:3351–61.
Hu L, Quach T, Han S, Lim SF, Yadav P, Senyschyn D, et al. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance Oral bioavailability. Angew Chemie. Wiley-Blackwell. 2016;128:13904–9.
Radwan AA, Alanazi FK. Targeting cancer using cholesterol conjugates. Saudi Pharm J Elsevier. 2014;22:3–16.
Radwan A, Alanazi F, Radwan AA, Alanazi FK. Design and synthesis of new cholesterol-conjugated 5-fluorouracil: a novel potential delivery system for cancer treatment. Molecules Multidisciplinary Digital Publishing Institute. 2014;19:13177–87.
Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol Nature Publishing Group. 2007;25:1149–57.
Dahan A, Duvdevani R, Shapiro I, Elmann A, Finkelstein E, Hoffman A. The oral absorption of phospholipid prodrugs: in vivo and in vitro mechanistic investigation of trafficking of a lecithin-valproic acid conjugate following oral administration. J Control Release Elsevier. 2008;126:1–9.
Labiner DM. DP-VPA D-Pharm. Curr Opin Investig Drugs. 2002;3:921–3.
Isoherranen N, Yagen B, Bialer M. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet? Curr Opin Neurol. 2003;16:203–11.
Bialer M, Johannessen S, Kupferberg H, Levy R, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Sixth EILAT Conference (EILAT VI). Epilepsy Res. Elsevier. 2002;51:31–71.
Bialer M, Johannessen S, Kupferberg H, Levy R, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Fifth EILAT Conference (EILAT V). Epilepsy Res Elsevier. 2001;43:11–58.
Dahan A, Duvdevani R, Dvir E, Elmann A, Hoffman A. A novel mechanism for oral controlled release of drugs by continuous degradation of a phospholipid prodrug along the intestine: in-vivo and in-vitro evaluation of an indomethacin–lecithin conjugate. J Control Release. Elsevier. 2007;119:86–93.
Dahan A, Markovic M, Epstein S, Cohen N, Zimmermann EM, Aponick A, et al. Phospholipid-drug conjugates as a novel oral drug targeting approach for the treatment of inflammatory bowel disease. Eur J Pharm Sci Elsevier. 2017;108:78–85.
Thanki K, Prajapati R, Sangamwar AT, Jain S. Long chain fatty acid conjugation remarkably decreases the aggregation induced toxicity of amphotericin. B. Int J Pharm. Elsevier. 2018;544:1–13 Available from: https://www.sciencedirect.com/science/article/pii/S0378517318302205. Accessed 19 Sept 2018.
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomed Nanotechnol Biol Med. Elsevier. 2018;14:1629–41 Available from: https://www.sciencedirect.com/science/article/pii/S1549963418300819. Accessed 19 Sept 2018.
Olbrich C, Gessner A, Kayser O, Müller RH. Lipid-drug-conjugate (ldc) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J Drug Target. 2002;10:387–96 Available from: http://www.tandfonline.com/doi/full/10.1080/1061186021000001832. Accessed 19 Sept 2018.
Wissing S, Kayser O, Müller R. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. Elsevier. 2004;56:1257–72 Available from: https://www.sciencedirect.com/science/article/pii/S0169409X04000456. Accessed 19 Sept 2018.
Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov Nature Publishing Group. 2015;14:781–803.
Braess J, Freund M, Hanauske A, Heil G, Kaufmann C, Kern W, et al. Oral cytarabine ocfosfate in acute myeloid leukemia and non-Hodgkin’s lymphoma—phase I/II studies and pharmacokinetics. Leukemia Nature Publishing Group. 1998;12:1618–26.
Saneyoshi M, Morozumi M, Kodama K, Machida H, Kuninaka A, Yoshino H. Synthetic nucleosides and nucleotides. XVI. Synthesis and biological evaluations of a series of 1-.BETA.-D-arabinofuranosylcytosine 5′-alkyl or arylphosphates. Chem Pharm Bull (Tokyo). The Pharmaceutical Society of Japan. 1980;28:2915–23.
Borkar N, Li B, Holm R, Håkansson AE, Müllertz A, Yang M, et al. Lipophilic prodrugs of apomorphine I: preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur J Pharm Biopharm Elsevier. 2015;89:216–23.
Bala V, Rao S, Li P, Wang S, Prestidge CA. Lipophilic prodrugs of SN38: synthesis and in vitro characterization toward oral chemotherapy. Mol Pharm. American Chemical Society. 2016;13:287–94.
Fleisher D, Bong R, Stewart BH. Improved oral drug delivery: solubility limitations overcome by the use of prodrugs. Adv Drug Deliv Rev. Elsevier. 1996;19:115–30.
You Y-J, Kim Y, Nam N-H, Ahn B-Z. Antitumor activity of unsaturated fatty acid esters of 4′-demethyldeoxypodophyllotoxin. Bioorg Med Chem Lett Pergamon. 2003;13:2629–32.
Naesens L, Neyts J, Balzarini J, Bischofberger N, De Clercq E. In vivo antiretroviral efficacy of oral bis(POM)-PMEA, the bis(pivaloyloxymethyl)prodrug of 9-(2-phosphonylmethoxyethyl) adenine (PMEA). Nucleosides Nucleotides Nucleic Acids. 1995;14:767–70.
Wichitnithad W, Nimmannit U, Wacharasindhu S, Rojsitthisak P, Wichitnithad W, Nimmannit U, et al. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules Molecular Diversity Preservation International. 2011;16:1888–900.
Charman WN, Porter CJH. Lipophilic prodrugs designed for intestinal lymphatic transport. Adv Drug Deliv Rev. 1996;19:149–69.
Sugihara J, Furuuchi S, Ando H, Takashima K, Harigaya S. Studies on intestinal lymphatic absorption of drugs. II. Glyceride prodrugs for improving lymphatic absorption of naproxen and nicotinic acid. J Pharmacobiodyn The Pharmaceutical Society of Japan. 1988;11:555–62.
Kumar R, Billimoria JD. Gastric ulceration and the concentration of salicylate in plasma in rats after administration of 14C-labelled aspirin and its synthetic triglyceride, 1,3-dipalmitoyl-2(2′-acetoxy-[14C]carboxylbenzoyl) glycerol. J Pharm Pharmacol. Wiley/Blackwell (10.1111). 1978;30:754–8.
Paris GY, Garmaise DL, Cimon DG, Swett L, Carter GW, Young P. Glycerides as prodrugs. 3. Synthesis and antiinflammatory activity of [1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetyl]glycerides (indomethacin glycerides). J Med Chem Am Chem Soc. 1980;23:9–13.
Paris GY, Garmaise DL, Cimon DG, Swett L, Carter GW, Young P. Glycerides as prodrugs. 2. 1,3-Dialkanoyl-2-(2-methyl-4-oxo-1,3-benzodioxan-2-yl)glycerides (cyclic aspirin triglycerides) as antiinflammatory agents. J Med Chem Am Chem Soc. 1980;23:79–82.
Hanauer SB. Review article: high-dose aminosalicylates to induce and maintain remissions in ulcerative colitis. Aliment Pharmacol Ther. Wiley/Blackwell (10.1111). 2006;24:37–40.
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. JNCI: Journal of the National Cancer Institute. 2015;107(2).
Fumagalli G, Marucci C, Christodoulou MS, Stella B, Dosio F, Passarella D. Self-assembly drug conjugates for anticancer treatment. Drug Discov Today. Elsevier Current Trends. 2016;21:1321–9.
Reddy LH, Marque P-E, Dubernet C, Mouelhi S-L, Desmaële D, Couvreur P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J Pharmacol Exp Ther American Society for Pharmacology and Experimental Therapeutics. 2008;325:484–90.
Kawabata K, Takakura Y, Hashida M. The fate of plasmid dna after intravenous injection in mice: involvment of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–30 Available from: https://link.springer.com/article/10.1023/A:1016248701505. Accessed 19 Sept 2018.
Gupta A, Asthana S, Konwar R, Chourasia MK. An insight into potential of nanoparticles-assisted chemotherapy of cancer using gemcitabine and its fatty acid prodrug: a comparative study. J Biomed Nanotechnol. 2013;9:915–25.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63.
Chue P, Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383–97.
ARISTADA® (aripiprazole lauroxil) | Every 2 Months (1064 mg) [Internet]. [cited 2018 Sep 15]. Available from: https://www.aristada.com/. Accessed 19 Sept 2018.
Meltzer HY, Risinger R, Nasrallah HA, Du Y, Zummo J, Corey L, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76:1085–90.
Rautio J, Kärkkäinen J, Sloan KB. Prodrugs—recent approvals and a glimpse of the pipeline. Eur J Pharm Sci. Elsevier B.V. 2017;109:146–61.
Hanaoka K, Suzuki M, Kobayashi T, Tanzawa F, Tanaka K, Shibayama T, et al. Antitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-?-d-ARABINO-Pentofuranosyl) cytosine) and itsN4-palmitoyl derivative (CS-682). Int J Cancer Wiley-Blackwell. 1999;82:226–36.
Painter GR, Almond MR, Trost LC, Lampert BM, Neyts J, De Clercq E, et al. Evaluation of hexadecyloxypropyl-9-R-[2-(Phosphonomethoxy)propyl]- adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob Agents Chemother. American Society for Microbiology Journals. 2007;51:3505–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Date, T., Paul, K., Singh, N. et al. Drug–Lipid Conjugates for Enhanced Oral Drug Delivery. AAPS PharmSciTech 20, 41 (2019). https://doi.org/10.1208/s12249-018-1272-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1272-0