Abstract
As the process analytical technology (PAT) mindset is progressively introduced and adopted by the pharmaceutical companies, there is an increasing demand for effective and versatile real-time analyzers to address the quality assurance challenges of drug manufacturing. In the last decades, Raman spectroscopy has emerged as one of the most promising tools for non-destructive and fast characterization of the pharmaceutical processes. This review summarizes the achieved results of the real-time application of Raman spectroscopy in the field of the secondary manufacturing of pharmaceutical solid dosage forms, covering the most common secondary process steps of a tablet production line. In addition, the feasibility of Raman spectroscopy for real-time control is critically reviewed, and challenges and possible approaches to moving from real-time monitoring to process analytically controlled technologies (PACT) are discussed.




Similar content being viewed by others
References
Food and Drug Administration. Guidance for industry: PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf Accessed 29 June 2018.
Sasic S, Ekins S. Pharmaceutical applications of Raman spectroscopy: Wiley; 2008.
Bakeev KA. Process analytical technology: spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries: Wiley; 2010.
Teżyk M, Milanowski B, Ernst A, Lulek J. Recent progress in continuous and semi-continuous processing of solid oral dosage forms: a review. Drug Dev Ind Pharm. 2016;42(8):1195–214.
De Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417(1):32–47.
Rantanen J. Process analytical applications of Raman spectroscopy. J Pharm Pharmacol. 2007;59(2):171–7.
Esmonde-White KA, Cuellar M, Uerpmann C, Lenain B, Lewis IR. Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal Bioanal Chem. 2017;409(3):637–49.
Jung N, Windbergs M. Raman spectroscopy in pharmaceutical research and industry. Phys Sci Rev. 2018. https://doi.org/10.1515/psr-2017-0045.
Brillouin L. Diffusion de la lumière et des rayons X par un corps transparent homogène. Influence de l’agitation thermique. Ann Phys. 1922;17:88–122.
Smekal A. Zur Quantentheorie der dispersion. Naturwissenschaften. 1923;11(43):873–5.
Raman CV, Krishnan KS. A new type of secondary radiation. Nature. 1928;121:501–2.
Smith E, Dent G. Modern Raman spectroscopy: a practical approach: John Wiley & Sons; 2013.
Das RS, Agrawal YK. Raman spectroscopy: recent advancements, techniques and applications. Vib Spectrosc. 2011;57(2):163–76.
Vankeirsbilck T, Vercauteren A, Baeyens W, Van der Weken G, Verpoort F, Vergote G, et al. Applications of Raman spectroscopy in pharmaceutical analysis. TrAC Trends Anal Chem. 2002;21(12):869–77.
Placzek G. The Rayleigh and Raman scattering. Handbuch der Radiologie. 1934;Vol 6 Part 2.
Schawlow AL, Townes CH. Infrared and optical masers. Phys Rev. 1958;112(6):1940–9.
Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187(4736):493–4.
Wikstrom H, Lewis IR, Taylor LS. Comparison of sampling techniques for in-line monitoring using Raman spectroscopy. Appl Spectrosc. 2005;59(7):934–41.
Lewis IR, Lewis ML. Fiber-optic probes for Raman spectrometry. Handbook of vibrational spectroscopy: John Wiley & Sons, Ltd.; 2006.
Ines L, Sebastian D, Christoph K, Benjamin D, Jürgen P. Fiber optic probes for linear and nonlinear Raman applications—current trends and future development. Laser Photonics Rev. 2013;7(5):698–731.
Franzen L, Windbergs M. Applications of Raman spectroscopy in skin research—from skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv Drug Deliv Rev. 2015;89:91–104.
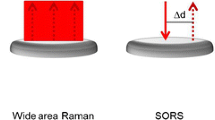
Kim M, Chung H, Woo Y, Kemper M. New reliable Raman collection system using the wide area illumination (WAI) scheme combined with the synchronous intensity correction standard for the analysis of pharmaceutical tablets. Anal Chim Acta. 2006;579(2):209–16.
Matousek P, Parker A. Bulk Raman analysis of pharmaceutical tablets. Appl Spectrosc. 2006;60(12):1353–7.
Matousek P, Parker AW. Non-invasive probing of pharmaceutical capsules using transmission Raman spectroscopy. J Raman Spectrosc. 2007;38(5):563–7.
Buckley K, Matousek P. Recent advances in the application of transmission Raman spectroscopy to pharmaceutical analysis. J Pharm Biomed Anal. 2011;55(4):645–52.
Allan P, Bellamy LJ, Nordon A, Littlejohn D, Andrews J, Dallin P. In situ monitoring of powder blending by non-invasive Raman spectrometry with wide area illumination. J Pharm Biomed Anal. 2013;76:28–35.
Lee S-H, Lee J-H, Cho S, Do S-H, Woo Y-A. End point determination of blending process for trimebutine tablets using principle component analysis (PCA) and partial least squares (PLS) regression. Arch Pharm Res. 2012;35(9):1599–607.
Vergote GJ, De Beer TRM, Vervaet C, Remon JP, Baeyens WRG, Diericx N, et al. In-line monitoring of a pharmaceutical blending process using FT-Raman spectroscopy. Eur J Pharm Sci. 2004;21(4):479–85.
Hausman DS, Cambron RT, Sakr A. Application of Raman spectroscopy for on-line monitoring of low dose blend uniformity. Int J Pharm. 2005;298(1):80–90.
Riolo D, Piazza A, Cottini C, Serafini M, Lutero E, Cuoghi E, et al. Raman spectroscopy as a PAT for pharmaceutical blending: advantages and disadvantages. J Pharm Biomed Anal. 2018;149(Supplement C):329–34.
De Beer TRM, Bodson C, Dejaegher B, Walczak B, Vercruysse P, Burggraeve A, et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J Pharm Biomed Anal. 2008;48(3):772–9.
Nagy B, Farkas A, Gyürkés M, Komaromy-Hiller S, Démuth B, Szabó B, et al. In-line Raman spectroscopic monitoring and feedback control of a continuous twin-screw pharmaceutical powder blending and tableting process. Int J Pharm. 2017;530(1):21–9.
McAuliffe MAP, O’Mahony GE, Blackshields CA, Collins JA, Egan DP, Kiernan L, et al. The use of PAT and off-line methods for monitoring of roller compacted ribbon and granule properties with a view to continuous processing. Org Process Res Dev. 2015;19(1):158–66.
Jørgensen A, Rantanen J, Karjalainen M, Khriachtchev L, Räsänen E, Yliruusi J. Hydrate formation during wet granulation studied by spectroscopic methods and multivariate analysis. Pharm Res. 2002;19(9):1285–91.
Wikström H, Marsac PJ, Taylor LS. In-line monitoring of hydrate formation during wet granulation using Raman spectroscopy. J Pharm Sci. 2005;94(1):209–19.
Christensen NPA, Cornett C, Rantanen J. Role of excipients on solid-state properties of piroxicam during processing. J Pharm Sci. 2012;101(3):1202–11.
Reddy JP, Jones JW, Wray PS, Dennis AB, Brown J, Timmins P. Monitoring of multiple solvent induced form changes during high shear wet granulation and drying processes using online Raman spectroscopy. Int J Pharm. 2018;541(1):253–60.
Fonteyne M, Vercruysse J, Díaz DC, Gildemyn D, Vervaet C, Remon JP, et al. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm Dev Technol. 2013;18(1):85–97.
Fonteyne M, Soares S, Vercruysse J, Peeters E, Burggraeve A, Vervaet C, et al. Prediction of quality attributes of continuously produced granules using complementary pat tools. Eur J Pharm Biopharm. 2012;82(2):429–36.
Harting J, Kleinebudde P. Development of an in-line Raman spectroscopic method for continuous API quantification during twin-screw wet granulation. Eur J Pharm Biopharm. 2018;125:169–81.
Hausman DS, Cambron RT, Sakr A. Application of on-line Raman spectroscopy for characterizing relationships between drug hydration state and tablet physical stability. Int J Pharm. 2005;299(1):19–33.
Otaki T, Tanabe Y, Kojima T, Miura M, Ikeda Y, Koide T, et al. In situ monitoring of cocrystals in formulation development using low-frequency Raman spectroscopy. Int J Pharm. 2018;542(1):56–65.
Walker GM, Bell SEJ, Greene K, Jones DS, Andrews GP. Characterisation of fluidised bed granulation processes using in-situ Raman spectroscopy. Chem Eng Sci. 2009;64(1):91–8.
Almeida A, Saerens L, De Beer T, Remon JP, Vervaet C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. Int J Pharm. 2012;439(1):223–9.
Saerens L, Ghanam D, Raemdonck C, Francois K, Manz J, Krüger R, et al. In-line solid state prediction during pharmaceutical hot-melt extrusion in a 12 mm twin screw extruder using Raman spectroscopy. Eur J Pharm Biopharm. 2014;87(3):606–15.
Saerens L, Segher N, Vervaet C, Remon JP, De Beer T. Validation of an in-line Raman spectroscopic method for continuous active pharmaceutical ingredient quantification during pharmaceutical hot-melt extrusion. Anal Chim Acta. 2014;806:180–7.
Netchacovitch L, Thiry J, De Bleye C, Dumont E, Cailletaud J, Sacré PY, et al. Global approach for the validation of an in-line Raman spectroscopic method to determine the API content in real-time during a hot-melt extrusion process. Talanta. 2017;171:45–52.
Saerens L, Vervaet C, Remon J-P, De Beer T. Visualization and process understanding of material behavior in the extrusion barrel during a hot-melt extrusion process using Raman spectroscopy. Anal Chem. 2013;85(11):5420–9.
Griffen JA, Owen AW, Matousek P. Quantifying low levels (< 0.5% w/w) of warfarin sodium salts in oral solid dose forms using transmission Raman spectroscopy. J Pharm Biomed Anal. 2018;155:276–83.
Townshend N, Nordon A, Littlejohn D, Myrick M, Andrews J, Dallin P. Comparison of the determination of a low-concentration active ingredient in pharmaceutical tablets by backscatter and transmission Raman spectrometry. Anal Chem. 2012;84(11):4671–6.
Gómez DA, Coello J, Maspoch S. Raman spectroscopy for the analytical quality control of low-dose break-scored tablets. J Pharm Biomed Anal. 2016;124:207–15.
Peeters E, Tavares da Silva AF, Toiviainen M, Van Renterghem J, Vercruysse J, Juuti M, et al. Assessment and prediction of tablet properties using transmission and backscattering Raman spectroscopy and transmission NIR spectroscopy. Asian J Pharm Sci. 2016;11(4):547–58.
Casian T, Reznek A, Vonica-Gligor AL, Van Renterghem J, De Beer T, Tomuță I. Development, validation and comparison of near infrared and Raman spectroscopic methods for fast characterization of tablets with amlodipine and valsartan. Talanta. 2017;167:333–43.
Virtanen S, Antikainen O, Yliruusi J. Determination of the crushing strength of intact tablets using Raman spectroscopy. Int J Pharm. 2008;360(1):40–6.
Nagy B, Farkas A, Magyar K, Démuth B, Nagy ZK, Marosi G. Spectroscopic characterization of tablet properties in a continuous powder blending and tableting process. Eur J Pharm Sci. 2018;123:10–9.
Kim B, Woo Y-A. Coating process optimization through in-line monitoring for coating weight gain using Raman spectroscopy and design of experiments. J Pharm Biomed Anal. 2018;154:278–84.
Hisazumi J, Kleinebudde P. In-line monitoring of multi-layered film-coating on pellets using Raman spectroscopy by MCR and PLS analyses. Eur J Pharm Biopharm. 2017;114:194–201.
Barimani S, Kleinebudde P. Evaluation of in-line Raman data for end-point determination of a coating process: comparison of science-based calibration, PLS-regression and univariate data analysis. Eur J Pharm Biopharm. 2017;119:28–35.
Romero-Torres S, Pérez-Ramos JD, Morris KR, Grant ER. Raman spectroscopic measurement of tablet-to-tablet coating variability. J Pharm Biomed Anal. 2005;38(2):270–4.
Nikowitz K, Folttmann F, Wirges M, Knop K, Pintye-Hódi K, Regdon G, et al. Development of a Raman method to follow the evolution of coating thickness of pellets. Drug Dev Ind Pharm. 2014;40(8):1005–10.
Kauffman JF, Dellibovi M, Cunningham CR. Raman spectroscopy of coated pharmaceutical tablets and physical models for multivariate calibration to tablet coating thickness. J Pharm Biomed Anal. 2007;43(1):39–48.
Barimani S, Kleinebudde P. Monitoring of tablet coating processes with colored coatings. Talanta. 2018;178:686–97.
Müller J, Knop K, Thies J, Uerpmann C, Kleinebudde P. Feasibility of Raman spectroscopy as PAT tool in active coating. Drug Dev Ind Pharm. 2010;36(2):234–43.
Müller J, Knop K, Wirges M, Kleinebudde P. Validation of Raman spectroscopic procedures in agreement with ICH guideline Q2 with considering the transfer to real time monitoring of an active coating process. J Pharm Biomed Anal. 2010;53(4):884–94.
El Hagrasy AS, Chang S-Y, Desai D, Kiang S. Raman spectroscopy for the determination of coating uniformity of tablets: assessment of product quality and coating pan mixing efficiency during scale-up. J Pharm Innov. 2006;1(1):37–42.
Wirges M, Funke A, Serno P, Knop K, Kleinebudde P. Development and in-line validation of a process analytical technology to facilitate the scale up of coating processes. J Pharm Biomed Anal. 2013;78–79:57–64.
Bogomolov A, Engler M, Melichar M, Wigmore A. In-line analysis of a fluid bed pellet coating process using a combination of near infrared and Raman spectroscopy. J Chemom. 2010;24(7–8):544–57.
Barimani S, Šibanc R, Kleinebudde P. Optimization of a semi-batch tablet coating process for a continuous manufacturing line by design of experiments. Int J Pharm. 2018;539(1):95–103.
Chakravarty P, Bhardwaj SP, King L, Suryanarayanan R. Monitoring phase transformations in intact tablets of trehalose by FT-Raman spectroscopy. AAPS PharmSciTech. 2009;10(4):1420–6.
Takeshima R, Hattori Y, Managaki S, Otsuka M. Analysis of the dehydration process of caffeine using backscattering and transmission Raman spectroscopy. Int J Pharm. 2017;530(1):256–62.
Eliasson C, Matousek P. Noninvasive authentication of pharmaceutical products through packaging using spatially offset Raman spectroscopy. Anal Chem. 2007;79(4):1696–701.
Lyndgaard LB, van den Berg F, de Juan A. Quantification of paracetamol through tablet blister packages by Raman spectroscopy and multivariate curve resolution-alternating least squares. Chemom Intell Laby Syst. 2013;125(Complete):58–66.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83.
Cullen PJ, RomaÃnach RJ, Abatzoglou N, Rielly CD. Pharmaceutical blending and mixing: John Wiley & Sons; 2015.
Fonteyne M, Vercruysse J, De Leersnyder F, Van Snick B, Vervaet C, Remon JP, et al. Process analytical technology for continuous manufacturing of solid-dosage forms. TrAC Trends Anal Chem. 2015;67:159–66.
Suresh P, Sreedhar I, Vaidhiswaran R, Venugopal A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem Eng J. 2017;328(Supplement C):785–815.
Burggraeve A, Monteyne T, Vervaet C, Remon JP, Beer TD. Process analytical tools for monitoring, understanding, and control of pharmaceutical fluidized bed granulation: a review. Eur J Pharm Biopharm. 2013;83(1):2–15.
Thompson MR, Sun J. Wet granulation in a twin-screw extruder: implications of screw design. J Pharm Sci. 2010;99(4):2090–103.
Hitzer P, Bäuerle T, Drieschner T, Ostertag E, Paulsen K, van Lishaut H, et al. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal Bioanal Chem. 2017;409(18):4321–33.
Saerens L, Vervaet C, Remon JP, De Beer T. Process monitoring and visualization solutions for hot-melt extrusion: a review. J Pharm Pharmacol. 2014;66(2):180–203.
De Leersnyder F, Peeters E, Djalabi H, Vanhoorne V, Van Snick B, Hong K, et al. Development and validation of an in-line NIR spectroscopic method for continuous blend potency determination in the feed frame of a tablet press. J Pharm Biomed Anal. 2018;151:274–83.
Karande AD, Heng PWS, Liew CV. In-line quantification of micronized drug and excipients in tablets by near infrared (NIR) spectroscopy: real time monitoring of tabletting process. Int J Pharm. 2010;396(1):63–74.
Järvinen K, Hoehe W, Järvinen M, Poutiainen S, Juuti M, Borchert S. In-line monitoring of the drug content of powder mixtures and tablets by near-infrared spectroscopy during the continuous direct compression tableting process. Eur J Pharm Sci. 2013;48(4–5):680–8.
Knop K, Kleinebudde P. PAT-tools for process control in pharmaceutical film coating applications. Int J Pharm. 2013;457(2):527–36.
Korasa K, Vrečer F. Overview of PAT process analysers applicable in monitoring of film coating unit operations for manufacturing of solid oral dosage forms. Eur J Pharm Sci. 2018;111:278–92.
Wulff R, Leopold CS. Coatings of Eudragit® RL and L-55 blends: investigations on the drug release mechanism. AAPS PharmSciTech. 2016;17(2):493–503.
da Silva CAM, Butzge JJ, Nitz M, Taranto OP. Monitoring and control of coating and granulation processes in fluidized beds—a review. Adv Powder Technol. 2014;25(1):195–210.
European Medical Agency. 2012. Guideline on real time release testing (formerly Guideline on parametric release). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/04/WC500125401.pdf. Accessed 29 June 2018.
Pestieau A, Krier F, Thoorens G, Dupont A, Chavez P-F, Ziemons E, et al. Towards a real time release approach for manufacturing tablets using NIR spectroscopy. J Pharm Biomed Anal. 2014;98(Supplement C):60–7.
Vargas JM, Nielsen S, Cárdenas V, Gonzalez A, Aymat EY, Almodovar E, et al. Process analytical technology in continuous manufacturing of a commercial pharmaceutical product. Int J Pharm. 2018;538(1–2):167–78.
Kourti T. Process analytical technology beyond real-time analyzers: the role of multivariate analysis. Crit Rev Anal Chem. 2006;36(3–4):257–78.
Singh R, Sahay A, Muzzio F, Ierapetritou M, Ramachandran R. A systematic framework for onsite design and implementation of a control system in a continuous tablet manufacturing process. Comput Chem Eng. 2014;66:186–200.
SIMATIC SIPAT. The software heart of PAT; White paper. Siemens AG.
Markl D, Wahl PR, Menezes JC, Koller DM, Kavsek B, Francois K, et al. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech. 2013;14(3):1034–44.
Nicolaï N, De Leersnyder F, Copot D, Stock M, Ionescu CM, Gernaey KV, et al. Liquid-to-solid ratio control as an advanced process control solution for continuous twin-screw wet granulation. AICHE J. 2018;64:2500–14.
Zacour BM, Drennen JK, Anderson CA. Development of a statistical tolerance-based fluid bed drying design space. J Pharm Innov. 2012;7(3):151–62.
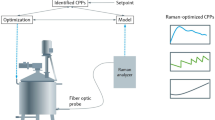
Csontos I, Pataki H, Farkas A, Bata H, Vajna B, Nagy ZK, et al. Feedback control of oximation reaction by inline Raman spectroscopy. Org Process Res Dev. 2015;19(1):189–95.
Djuris J, Djuric Z. Modeling in the quality by design environment: regulatory requirements and recommendations for design space and control strategy appointment. Int J Pharm. 2017;533(2):346–56.
Matero S, Fv DB, Poutiainen S, Rantanen J, Pajander J. Towards better process understanding: chemometrics and multivariate measurements in manufacturing of solid dosage forms. J Pharm Sci. 2013;102(5):1385–403.
Chen Z, Lovett D, Morris J. Process analytical technologies and real time process control a review of some spectroscopic issues and challenges. J Process Control. 2011;21(10):1467–82.
Pataki H, Csontos I, Nagy ZK, Vajna B, Molnar M, Katona L, et al. Implementation of Raman signal feedback to perform controlled crystallization of carvedilol. Org Process Res Dev. 2013;17(3):493–9.
Hirsch E, Pataki H, Farkas A, Bata H, Vass P, Fehér C, et al. Raman-based feedback control of the enzymatic hydrolysis of lactose. Org Process Res Dev. 2016;20(10):1721–7.
Matthews TE, Berry BN, Smelko J, Moretto J, Moore B, Wiltberger K. Closed loop control of lactate concentration in mammalian cell culture by Raman spectroscopy leads to improved cell density, viability, and biopharmaceutical protein production. Biotechnol Bioeng. 2016;113(11):2416–24.
Craven S, Whelan J, Glennon B. Glucose concentration control of a fed-batch mammalian cell bioprocess using a nonlinear model predictive controller. J Process Control. 2014;24(4):344–57.
Voss J-P, Mittelheuser NE, Lemke R, Luttmann R. Advanced monitoring and control of pharmaceutical production processes with Pichia pastoris by using Raman spectroscopy and multivariate calibration methods. Eng Life Sci. 2017;17(12):1281–94.
Singh R, Barrasso D, Chaudhury A, Sen M, Ierapetritou M, Ramachandran R. Closed-loop feedback control of a continuous pharmaceutical tablet manufacturing process via wet granulation. J Pharm Innov. 2014;9(1):16–37.
Singh R, Sahay A, Karry KM, Muzzio F, Ierapetritou M, Ramachandran R. Implementation of an advanced hybrid MPC–PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. Int J Pharm. 2014;473(1–2):38–54.
Hattori Y, Otsuka M. Modeling of feed-forward control using the partial least squares regression method in the tablet compression process. Int J Pharm. 2017;524(1):407–13.
Su Q, Moreno M, Giridhar A, Reklaitis GV, Nagy ZK. A systematic framework for process control design and risk analysis in continuous pharmaceutical solid-dosage manufacturing. J Pharm Innov. 2017;12(4):327–46.
Kourti T, Lepore J, Liesum L, Nasr M, Chatterjee S, Moore CM, et al. Scientific and regulatory considerations for implementing mathematical models in the quality by design (QbD) framework. Pharm Eng. 2014;34(6).
Funding
This work was supported by the National Research, Development, and Innovation Fund of Hungary in the frame of FIEK_16-1-2016-0007 (Higher Education and Industrial Cooperation Center) and KH 124541, GINOP-2.1.7-15-2016-01301. The authors also acknowledge the ÚNKP-18-2-I New National Excellence Program of the Ministry of Human Capacities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: William C. Stagner and Rahul V. Haware
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagy, B., Farkas, A., Borbás, E. et al. Raman Spectroscopy for Process Analytical Technologies of Pharmaceutical Secondary Manufacturing. AAPS PharmSciTech 20, 1 (2019). https://doi.org/10.1208/s12249-018-1201-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1201-2