Abstract
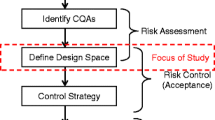
The primary objective of this study was to compare two methods for establishing a design space for critical process parameters that affect ethylcellulose film coating of multiparticulate beads and assess this design space validity across manufacturing scales. While there are many factors that can affect film coating, this study will focus on the effects processing conditions have on the quality and extent of film formation, as evaluated by their impact coating yield and drug release. Ciprofloxacin HCl layered beads were utilized as an active substrate core, ethylcellulose aqueous dispersion as a controlled release polymer, and triethyl citrate as a plasticizer. Thirty experiments were conducted using a central composite design to optimize the coating process and map the response surface to build a design space using either statistical least squares or a Bayesian approach. The response surface was fitted using a linear two-factor interaction model with spraying temperature, curing temperature, and curing time as significant model terms. The design spaces established by the two approaches were in close agreement with the statistical least squares approach being more conservative than the Bayesian approach. The design space established for the critical process parameters using small-scale batches was tested using scale-up batches and found to be scale-independent. The robustness of the design space was confirmed across scales and was successfully utilized to establish process signature for the coating process.














Similar content being viewed by others
References
Maejima T, McGinity JW. Influence of film additives on stabilizing drug release rates from pellets coated with acrylic polymers. Pharm Dev Technol. 2001;6(2):211–21.
Tabasi SH, Fahmy R, Bensley D, O'Brien C, Hoag SW. Quality by design, part I: application of NIR spectroscopy to monitor tablet manufacturing process. J Pharm Sci. 2008;97(9):4040–51. https://doi.org/10.1002/jps.21303.
Tabasi SH, Fahmy R, Bensley D, O'Brien C, Hoag SW. Quality by design, part II: application of NIR spectroscopy to monitor the coating process for a pharmaceutical sustained release product. J Pharm Sci. 2008;97(9):4052–66. https://doi.org/10.1002/jps.21307.
Tabasi SH, Fahmy R, Bensley D, O'Brien C, Hoag SW. Quality by design, part III: study of curing process of sustained release coated products using NIR spectroscopy. J Pharm Sci. 2008;97(9):4067–86. https://doi.org/10.1002/jps.21420.
Visschers M, Laven J, German AL. Current understanding of the deformation of latex particles during film formation. Prog Org Coat. 1997;30(1–2):39–49. https://doi.org/10.1016/S0300-9440(96)00652-2.
Keddie JL. Film formation of latex. Mater Sci Eng. 1997;21:101–70.
Steward PA, Hearn J, Wilkinson MC. An overview of polymer latex film formation and properties. Adv Colloid Interf Sci. 2000;86(3):195–267. https://doi.org/10.1016/S0001-8686(99)00037-8.
Wicks ZW. Free volume and the coatings formulator. J Coatings Technol. 1986;58:23–32.
Siepmann J, Lecomte F, Bodmeier R. Diffusion-controlled drug delivery systems: calculation of the required composition to achieve desired release profiles. J Control Release. 1999;60(2–3):379–89. https://doi.org/10.1016/s0168-3659(99)00093-0.
Yang QW, Flament MP, Siepmann F, Busignies V, Leclerc B, Herry C, et al. Curing of aqueous polymeric film coatings: importance of the coating level and type of plasticizer. Eur J Pharm Biopharm. 2010;74(2):362–70. https://doi.org/10.1016/j.ejpb.2009.10.007.
Korber M, Hoffart V, Walther M, Macrae RJ, Bodmeier R. Effect of unconventional curing conditions and storage on pellets coated with Aquacoat ECD. Drug Dev Ind Pharm. 2010;36(2):190–9. https://doi.org/10.3109/03639040902882314.
Hutchings D, Kuzmak B, Sakr A. Processing considerations for an EC latex coating system: influence of curing time and temperature. Pharm Res. 1994;11(10):1474–8. https://doi.org/10.1023/a:1018960310144.
Kothari BH, Fahmy R, Claycamp HG, Moore CMV, Chatterjee S, Hoag SW. A systematic approach of employing quality by design principles: risk assessment and design of experiments to demonstrate process understanding and identify the critical process parameters for coating of the ethylcellulose pseudolatex dispersion using non-conventional fluid bed process. AAPS PharmSciTech. 2016;18:1–23. https://doi.org/10.1208/s12249-016-0569-0.
Muliadi AR, Sojka PE. A review of pharmaceutical tablet spray coating. 2010;20(7):611–38. https://doi.org/10.1615/AtomizSpr.v20.i7.40.
Jones DM. Coating process and equipment. In: Hoag SW, Augsburger LL, editors. Pharmaceutical dosage forms: tablets. New York: Informa Healthcare; 2008. p. 379–97.
Kukec S, Vrecer F, Dreu R. A study of in situ fluid bed melt granulation using response surface methodology / Uporaba metodologije odgovornih povrsin za studij in situ granulacije s talinami v zvrtincenih plasteh. Acta Pharma. 2012;62(4):497–513. https://doi.org/10.2478/v10007-012-0033-y.
Vanaja K, Shobha Rani RH. Design of experiments: concept and applications of Plackett Burman design. Clin Res Regul Aff. 2007;24(1):1–23. https://doi.org/10.1080/10601330701220520.
Rambali BB,L, Massart DL. Using experimental design to optimize the process parameters in fluidized bed granulation on a semi-full scale. Int J Pharm. 2001;220:149–60.
Carley K, Kamneva N, Reminga J. Response surface methodology. Center for Computational Analysis of Social and Organistional Systems. 2004; CASOS Technical Report.
Peterson JJ. A Bayesian approach to the ICH Q8 definition of design space. J Biopharm Stat. 2008;18(5):959–75. https://doi.org/10.1080/10543400802278197.
Marucci M, Holmgren A, Carlsson H, Jarke A, Johansson M, Corswant C. Non-uniformity of pellets coating, effect on the dose release profile and how to improve the coating process by reducing the electrostatic charging of the pellets. Chem Biochem Eng Q. 2012;26(4):379–84.
Rhodes CT, Porter SC. Coatings for controlled-release drug delivery systems. Drug Dev Ind Pharm. 1998;24(12):1139–54. https://doi.org/10.3109/03639049809108573.
Melegari C, Bertoni S, Genovesi A, Hughes K, Rajabi-Siahboomi AR, Passerini N, et al. Ethylcellulose film coating of guaifenesin-loaded pellets: a comprehensive evaluation of the manufacturing process to prevent drug migration. Eur J Pharm Biopharm. 2016;100:15–26. https://doi.org/10.1016/j.ejpb.2015.12.001.
Dejaegher B, Heyden YV. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J Pharm Biomed Anal. 2011;56(2):141–58. https://doi.org/10.1016/j.jpba.2011.04.023.
Zhang Z, Xiaofeng B. Comparison about the three central composite designs with simulation. Proceedings of the 2009 International Conference on Advanced Computer Control: IEEE Computer Society; 2009. pp 163–7.
Whitcomb PJ. FDS—powerfull tool for response surface design. Stat-Teaser, News from Stat-Ease, Inc. 2008;(1).
Peterson JJ, Yahyah M. A Bayesian design space approach to robustness and system suitability for pharmaceutical assays and other processes. Stat Biopharm Res. 2009;1(4):441–9. https://doi.org/10.1198/sbr.2009.0037.
Box G, Hunter J. Multi-factor experimental designs for exploring response surfaces. Ann Math Stat. 1957;28(1):195–241.
Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc. 1962;57(298):248–368.
R. A language environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011.
SAS OnlineDoc(R) 9.3. Cary, NC: SAS Institute Inc; 2011.
Rossi P. Bayesm: Bayesian inference for marketing/micro-economics. R package version 2.2–4. Rossi, P. perossichi@gmail.com; 2011.
Casella G, George E. Explaining the Gibbs sampler. Am Stat. 1992;46(3):167–74.
Griffiths W, Valenzuela M. Gibbs sampler for a set of seemingly unrelated regressions. Aust N Z J Stat. 2006;48(3):335–51.
Robert C, Casella G. Introducing Monte Carlo methods in R. New York: Springer Science+Business Media; 2010.
Box G, Hunter J. A confidence region for the solution of a set of simultaneous equations with an application to experimental design. Biometrika. 1954;41:190–9.
Peterson JJ. A general approach to ridge analysis with confidence intervals. Technometrics. 1993;35(2):204–14.
Acknowledgements
The authors would like to thank Dr. Salah Ahmed, CEO of Abon Pharmaceuticals for allowing us to conduct scale-up experiments at their facility, Dr. Brian Carlin and Dr. Rina Choksi from FMC Corp. for their participation in the project, and Oystar Huttlin, Germany for providing the fluid bed Mycrolab at the University of Maryland, Bela Janscik from OPULUS for supplying the Pyrobutton package. The content of this paper was part of the graduate thesis dissertation submitted by Bhaveshkumar H. Kothari to the faculty of the School of Pharmacy, University of Maryland Baltimore in partial fulfillment of the requirements for the doctorate degree in Pharmaceutical Sciences—2013.
Funding
Funding for the project was from US Food and Drug Administration (FDA) under grant no. HHSF223201110076A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kothari, B.H., Fahmy, R., Claycamp, H.G. et al. Comparing a Statistical Model and Bayesian Approach to Establish the Design Space for the Coating of Ciprofloxacin HCl Beads at Different Scales of Production. AAPS PharmSciTech 19, 3809–3828 (2018). https://doi.org/10.1208/s12249-018-1116-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1116-y