Abstract
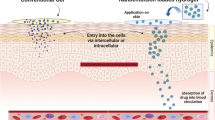
Zein is a naturally occurring corn protein having similarity to skin keratin. Owing to its hydrophobicity and biodegradability, zein nanocarriers are promising drug delivery vehicles for hydrophobic dermatological drugs. In this study, zein-based nanocapsules (ZNCs) were exploited for the first time as dermal delivery carriers for flutamide (FLT), an antiandrogen used for the management of pilosebasceous unit disorders. FLT-loaded ZNC of appropriate particle size and negative surface charge were prepared by nanoprecipitation method. The dermal permeation and skin retention of FLT from ZNCs were studied in comparison to corresponding nanoemulsion (NE) and hydroalcoholic drug solution (HA). ZNCs showed a significantly lower permeation flux compared to NE and HA while increasing the skin retention of FLT. Confocal laser scanning microscopy (CLSM) demonstrated the follicular localization of the fluorescently labeled NCs. The incorporation of NCs in chitosan gel or Carbomer® 934 gel was studied. Carbomer® gel increased the skin retention of FLT compared to chitosan gel. Accordingly, Carbomer® hydrogel embedding FLT-loaded ZNCs is a promising inexpensive, biocompatible dermal delivery nanocarrier for localized therapy of PSU disorders suitable for application on oily skin.








Similar content being viewed by others
References
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–72.
Knutsen-Larson S, Dawson AL, Dunnick CA, Dellavalle RP. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30(1):99–106.
Cash T. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141:398–405.
Husein-ElAhmed H. Management of acne vulgaris with hormonal therapies in adult female patients. Dermatol Ther. 2015;28(3):166–72.
Brufsky A, Fontaine-Rothe P, Berlane K, Rieker P, Jiroutek M, Kaplan I, et al. Finasteride and flutamide as potency-sparing androgen-ablative therapy for advanced adenocarcinoma of the prostate. Urology. 1997;49(6):913–20.
Adalatkhah H, Pourfarzi F, Sadeghi-Bazargani H. Flutamide versus a cyproterone acetate-ethinyl estradiol combination in moderate acne: a pilot randomized clinical trial. Clin Cosmet Investig Dermatol. 2011;4:117–21.
Paradisi R, Porcu E, Fabbri R, Seracchioli R, Battaglia C, Venturoli S. Prospective cohort study on the effects and tolerability of flutamide in patients with female pattern hair loss. Ann Pharmacother. 2011;45(4):469–75.
Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization stability and performance. Int J Pharm. 2011;414(1):267–75.
Durán N, Teixeira Z, Marcato PD. Topical application of nanostructures: solid lipid, polymeric and metallic nanoparticles. Nanocosmetics and Nanomedicines: Springer. 2011. p. 69–99.
Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2013;5(3):205–18.
Jain AK, Jain A, Garg NK, Agarwal A, Jain A, Jain SA, et al. Adapalene loaded solid lipid nanoparticles gel: an effective approach for acne treatment. Colloids Surf B: Biointerfaces. 2014;121:222–9.
Sallam MA, Marin Bosca MT. Mechanistic analysis of human skin distribution and follicular targeting of adapalene-loaded biodegradable Nanospheres with an insight into hydrogel matrix influence, in vitro skin irritation, and in vivo tolerability. J Pharm Sci. 2017;106(10):3140–9.
Madheswaran T, Baskaran R, Sundaramoorthy P, Yoo BK. Enhanced skin permeation of 5alpha-reductase inhibitors entrapped into surface-modified liquid crystalline nanoparticles. Arch Pharm Res. 2015;38(4):534–42.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385(1–2):113–42.
Poletto FS, Beck RC, Guterres SS, Pohlmann AR. Polymeric nanocapsules: concepts and applications. Nanocosmetics and nanomedicines: Springer; 2011. p. 49–68.
Ourique A, Pohlmann A, Guterres S, Beck R. Tretinoin-loaded nanocapsules: preparation, physicochemical characterization, and photostability study. Int J Pharm. 2008;352(1):1–4.
De Brum TL, Fiel LA, Contri RV, Guterres SS, Pohlmann AR. Polymeric nanocapsules and lipid-core nanocapsules have diverse skin penetration. J Nanosci Nanotechnol. 2015;15(1):773–80.
Santos SS, Lorenzoni A, Ferreira LM, Mattiazzi J, Adams AI, Denardi LB, et al. Clotrimazole-loaded Eudragit® RS100 nanocapsules: preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater Sci Eng C. 2013;33(3):1389–94.
Teixeira M, Alonso MJ, Pinto MM, Barbosa CM. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur J Pharm Biopharm. 2005;59(3):491–500.
Gaber M, Medhat W, Hany M, Saher N, Fang J-Y, Elzoghby A. Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: challenges and outcomes. J Control Release. 2017;254:75–91.
Elzoghby AO, Vranic BZ, Samy WM, Elgindy NA. Swellable floating tablet based on spray-dried casein nanoparticles: near-infrared spectral characterization and floating matrix evaluation. Int J Pharm. 2015;491(1):113–22.
Wang L, Qin G, Geng S, Dai Y, Wang J-Y. Preparation of zein conjugated quantum dots and their in vivo transdermal delivery capacity through nude mouse skin. J Biomed Nanotechnol. 2013;9(3):367–76.
Elzoghby AO, Elgohary MM, Kamel NM. Chapter six-implications of protein-and peptide-based nanoparticles as potential vehicles for anticancer drugs. Adv Protein Chem Struct Biol. 2015;98:169–221.
Muthuselvi L, Dhathathreyan A. Simple coacervates of zein to encapsulate gitoxin. Colloids Surf B: Biointerfaces. 2006;51(1):39–43.
Zhong Q, Tian H, Zivanovic S. Encapsulation of fish oil in solid zein particles by liquid-liquid dispersion. J Food Process Preserv. 2009;33(2):255–70.
Elzoghby AO, Helmy MW, Samy WM, Elgindy NA. Micellar delivery of flutamide via milk protein nanovehicles enhances its anti-tumor efficacy in androgen-dependent prostate cancer rat model. Pharm Res. 2013;30(10):2654–63.
Venturini CG, Jäger E, Oliveira CP, Bernardi A, Battastini AM, Guterres SS, et al. Formulation of lipid core nanocapsules. Colloids Surf A Physicochem Eng Asp. 2011;375(1):200–8.
Sallam MA, Helal HM, Mortada SM. Rationally designed nanocarriers for intranasaltherapy of allergic rhinitis: influence of carrier type on in vivo nasal deposition. Int J Nanomedicine. 2016;11:2345.
Sallam MA, Motawaa AM, Mortada SM. A modern approach for controlled transdermal delivery of diflunisal: optimization and in vivo evaluation. Drug Dev Ind Pharm. 2013;39(4):600–10.
Elzoghby A, Freag M, Mamdouh H, Elkhodairy K. Zein-based nanocarriers as potential natural alternatives for drug and gene delivery: focus on cancer therapy. Curr Pharm Des. 2017.
Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release. 2014;189:108–22.
Lai L, Guo H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int J Pharm. 2011;404(1):317–23.
Blouza IL, Charcosset C, Sfar S, Fessi H. Preparation and characterization of spironolactone-loaded nanocapsules for paediatric use. Int J Pharm. 2006;325(1):124–31.
Rodriguez-Emmenegger C, Jäger A, Jäger E, Stepanek P, Alles AB, Guterres S, et al. Polymeric nanocapsules ultra stable in complex biological media. Colloids Surf B: Biointerfaces. 2011;83(2):376–81.
Elzoghby AO, Mostafa SK, Helmy MW, ElDemellawy MA, Sheweita SA. Multi-reservoir phospholipid Shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm Res. 2017;34(9):1956–69.
Elzoghby AO, Mostafa SK, Helmy MW, ElDemellawy MA, Sheweita SA. Superiority of aromatase inhibitor and cyclooxygenase-2 inhibitor combined delivery: hyaluronate-targeted versus PEGylated protamine nanocapsules for breast cancer therapy. Int J Pharm. 2017;529(1–2):178–92.
Lobato KB, Paese K, Forgearini JC, Guterres SS, Jablonski A, Rios Ade O. Characterisation and stability evaluation of bixin nanocapsules. Food Chem. 2013;141(4):3906–12.
Elgindy N, Elkhodairy K, Molokhia A, Elzoghby A. Lyophilization monophase solution technique for preparation of amorphous flutamide dispersions. Drug Dev Ind Pharm. 2011;37(7):754–64.
Abdel-Mottaleb MM, Neumann D, Lamprecht A. Lipid nanocapsules for dermal application: a comparative study of lipid-based versus polymer-based nanocarriers. Eur J Pharm Biopharm. 2011;79(1):36–42.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37.
Contri R, Fiel L, Alnasif N, Pohlmann A, Guterres S, Schäfer-Korting M. Skin penetration and dermal tolerability of acrylic nanocapsules: influence of the surface charge and a chitosan gel used as vehicle. Int J Pharm. 2016;507(1):12–20.
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym. 2010;82(2):227–32.
Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol. 2014;64:353–67.
Anirudhan TS, Nair SS, Nair AS. Fabrication of a bioadhesive transdermal device from chitosan and hyaluronic acid for the controlled release of lidocaine. Carbohydr Polym. 2016;152:687–98.
Hafner A, Lovric J, Pepic I, Filipovic-Grcic J. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J Microencapsul. 2011;28(8):807–15.
Machida Y, Masuda H, Fujiyama N, Iwata M, Nagai T. Preparation and phase II clinical examination of topical dosage forms for the treatment of carcinoma colli containing bleomycin, carboquone, or 5-fluorouracil with Hydroxypropyl cellulose. Chem Pharm Bull (Tokyo). 1980;28(4):1125–30.
Shin SC, Kim HJ, Oh IJ, Cho CW, Yang KH. Development of tretinoin gels for enhanced transdermal delivery. Eur J Pharm Biopharm. 2005;60(1):67–71.
Park SN, Jo NR, Jeon SH. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J Ind Eng Chem. 2014;20(4):1481–5.
Taveira SF, Nomizo A, Lopez RF. Effect of the iontophoresis of a chitosan gel on doxorubicin skin penetration and cytotoxicity. J Control Release. 2009;134(1):35–40.
He W, Guo X, Xiao L, Feng M. Study on the mechanisms of chitosan and its derivatives used as transdermal penetration enhancers. Int J Pharm. 2009;382(1–2):234–43.
Yang Y, Sunoqrot S, Stowell C, Ji J, Lee C-W, Kim JW, et al. Effect of size, surface charge, and hydrophobicity of poly (amidoamine) dendrimers on their skin penetration. Biomacromolecules. 2012;13(7):2154–62.
Gillet A, Compère P, Lecomte F, Hubert P, Ducat E, Evrard B, et al. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 2011;411(1):223–31.
Lademann J, Patzelt A, Richter H, Antoniou C, Sterry W, Knorr F. Determination of the cuticula thickness of human and porcine hairs and their potential influence on the penetration of nanoparticles into the hair follicles. J Biomed Opt. 2009;14(2):021014.
Rancan F, Afraz Z, Combadiere B, Blume-Peytavi U, Vogt A. Hair follicle targeting with nanoparticles. In: Nasir A, Friedman A, Wang S, editors. Nanotechnology in dermatology. New York, NY: Springer New York; 2013. p. 95–107.
Acknowledgments
This work is supported by a research grant funded by center of special studies at bibliotheca Alexandrina (CSSP-BIBALEX).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors reported no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 169 kb)
Rights and permissions
About this article
Cite this article
Sallam, M.A., Elzoghby, A.O. Flutamide-Loaded Zein Nanocapsule Hydrogel, a Promising Dermal Delivery System for Pilosebaceous Unit Disorders. AAPS PharmSciTech 19, 2370–2382 (2018). https://doi.org/10.1208/s12249-018-1087-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1087-z