Abstract
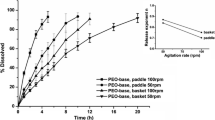
The USP Apparatus 3 is a compendial dissolution Apparatus that has been mainly used to assess the performance of modified-release drug products. However, this Apparatus can be applied to dissolution testing of immediate-release tablets as well, with several advantages such as lower consumption of dissolution media, reduced setup time in quality control routine, and minimized hydrodynamic issues. In this work, three immediate-release (IR) tablets containing antihypertensive drugs of different Biopharmaceutic Classification System (BCS) classes were evaluated in order to assess the possible interchangeability between the official dissolution method using typical USP Apparatus 1 or 2 and the proposed methods using USP Apparatus 3. Depending on the selection of the appropriate operational conditions, such as dip rate and sieve mesh size, it was observed that USP Apparatus 3 could provide similar dissolution profiles compared to USP Apparatus 1 or 2 to the drug products tested. In addition, USP Apparatus 3 avoided conning issues related to USP Apparatus 2. The successful application of USP Apparatus 3 in dissolution tests for IR drug products depends on the definition of specific test conditions for each product, considering all the equipment variables, as well as drug and formulation characteristics.






Similar content being viewed by others
References
Mwila C, Khamanga S, Walker R. Development and assessment of a USP Apparatus 3 dissolution test method for sustained-release nevirapine matrix tablets. Dissolut Technol. 2016;23(3):22–30. https://doi.org/10.14227/DT230316P22.
Cardot J-M, Beyssac E, Alric M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolut Technol. 2007;14(1):15–9. https://doi.org/10.14227/DT140107P15.
Hörter D, Dressman J. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 2001;46(1–3):75–87. https://doi.org/10.1016/S0169-409X(00)00130-7.
Shohin IE, Grebenkin DY, Malashenko EA, Stanishevskii YM, Ramenskaya GV. A brief review of the FDA dissolution methods database. Dissolut Technol 2016;23(3):6–10. https://doi.org/10.14227/DT230316P6.
Borst I, Ugwu S, Beckett AH. New and extended applications for USP drug release apparatus 3. Dissolut Technol. 1997;4(1):11–8. https://doi.org/10.14227/DT040197P11.
Pezzini BR, Issa MG, Duque MD, Ferraz HG. Applications of USP apparatus 3 in assessing the in vitro release of solid oral dosage forms. Braz J Pharm Sci. 2015;51(2):265–72. https://doi.org/10.1590/S1984-82502015000200003.
Yu LX, Wang JT, Hussain AS. Evaluation of USP apparatus 3 for dissolution testing of immediate-release products. AAPS PharmSci. 2002;4:1):1–5. https://doi.org/10.1208/ps040101.
Webster GK, Jackson JD, Bell RG. Poorly soluble drugs: dissolution and drug release. Pan Stanford; 2017. 686 p.
Crist GB. 2009 trends in small-volume dissolution apparatus for low-dose compounds. Dissolut Technol. 2009;16(1):19–22. https://doi.org/10.14227/DT160109P19.
Li J, Yang L, Ferguson SM, Hudson TJ, Watanabe S, Katsuma M, et al. In vitro evaluation of dissolution behavior for a colon-specific drug delivery system (CODES) in multi-pH media using United States Pharmacopeia apparatus II and III. AAPS PharmSciTech. 2002;3(4):E33–22. https://doi.org/10.14227/DT160109P19.
USP. THE UNITED STATES PHARMACOPEIA. 39th ed. USP39–NF34. Rockville: United States Pharmacopeial Convention; 2016. 8016 p.
Klein S, Rudolph MW, Dressman JB. Drug release characteristics of different mesalazine products using USP apparatus 3 to simulate passage through the GI tract. Dissolut Technol 2002;9(4):6–12. https://doi.org/10.14227/DT090402P6.
Cacace J, Reilly EE, Amann A. Comparison of the dissolution of metaxalone tablets (Skelaxin) usingUSP apparatus 2 and 3. AAPS PharmSciTech. 2004;5(1):29–31. https://doi.org/10.1208/pt050106.
Mercuri A, Fares R, Bresciani M, Fotaki N. An in vitro–in vivo correlation study for nifedipine immediate release capsules administered with water, alcoholic and non-alcoholic beverages: impact of in vitro dissolution media and hydrodynamics. Int J Pharm. 2016;499(1–2):330–42. https://doi.org/10.1016/j.ijpharm.2015.12.047.
Sanghvi PP, Nambiar JS, Shukla AJ, Collins CC. Comparison of three dissolution devices for evaluating drug release. Drug Dev Ind Pharm. 1994 Jan 20;20(6):961–80. https://doi.org/10.3109/03639049409038344.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. https://doi.org/10.1023/A:1016212804288.
Elder D, Holm R. Aqueous solubility: simple predictive methods (in silico, in vitro and bio-relevant approaches). Int J Pharm. 2013;453(1):3–11. https://doi.org/10.1016/j.ijpharm.2012.10.041.
Nart V. DESENVOLVIMENTO E AVALIAÇÃO DE SISTEMAS MULTIPARTICULADOS NA FORMA DE MINI Florianópolis. Universidade Federal de Santa Catarina; 2015.
Weich A, de Oliveira DC, de Melo J, Goebel K, Rolim CMB. Validation of UV spectrophotometric and HPLC methods for quantitative determination of atenolol in pharmaceutical preparations. Lat Am J Pharm. 2007;26(5):765–70.
CDER/FDA. Guidance for Industry Dissolution Testing of Immediate. Vol. 4, Center for Drug Evaluation and Research. 1997.
CDER/FDA. Guidance for Industry, Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system. Center for Drug Evaluation and Research. 2015.
EMA. Note for guidance on the investigation of bioavailability and bioequivalence. Committee for proprietary medicinal products. 2001.
Swami R, Singh G, Bhasin P, Dureja H. In vitro dissolution profile comparison: a tool for biowaivers based on BCS. J Pharm Res. 2011;10(2):73–6. https://doi.org/10.18579/jpcrkc/2011/10/2/89014.
Ozturk N, Kaynak MS, Sahin S. Comparison of dissolution profiles of commercially available lamivudine tablets. Dissolut Technol. 2015;22(4):38–43. https://doi.org/10.14227/DT220415P38.
Anderson NH, Bauer M, Boussac N, Khan-Malek R, Munden P, Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J Pharm Biomed Anal. 1998;17(4–5):811–22. https://doi.org/10.1016/S0731-7085(98)00011-9.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33. https://doi.org/10.1016/S0928-0987(01)00095-1.
Qiu Y, Chen Y, Zhang GGZ, Liu L, Porter W. Developing solid oral dosage forms: pharmaceutical theory & practice: Academic Press; 2009.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66. https://doi.org/10.1016/j.ejps.2013.08.024.
Seeger N, Lange S, Klein S. Impact of vibration and agitation speed on dissolution of USP prednisone tablets RS and various IR tablet formulations. AAPS PharmSciTech. 2015;16(4):759–66. https://doi.org/10.1208/s12249-015-0356-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editor: Sandra Klein
Rights and permissions
About this article
Cite this article
Espíndola, B., Bortolon, F.F., Pinto, J.M.O. et al. New Approach for the Application of USP Apparatus 3 in Dissolution Tests: Case Studies of Three Antihypertensive Immediate-Release Tablets. AAPS PharmSciTech 19, 2866–2874 (2018). https://doi.org/10.1208/s12249-018-1086-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1086-0