Abstract
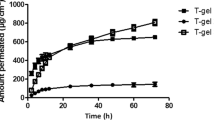
The adequate management of analgesia, by pharmacological methods or not, is a great challenge. Local anesthetics are used for pain relief, mainly by parenteral, intramuscular, catheter, and other routes of administration. The use of in situ forming systems becomes an alternative for the control of pain. The present research investigates development of thermogels containing poloxamer and levobupivacaine. All formulations were prepared by the cold method; the compatibilities of the excipients were evaluated by DSC, rheology and viscosities, transition temperature, syringeability, release kinetics, and permeation. The compatibility of the tested excipients with the drug was initially observed; all formulations had a viscosity increase at 37°C. Different delivery rates were observed in both the release and permeation studies. The developed systems maintained the in vitro release of the drug for a long period, likely decreasing side effects in vivo and avoiding the need for supplementary analgesia by other routes.




Similar content being viewed by others
References
Pimenta CADM, Santos EMM, Chaves LD, Martins LM, Gutierrez BAO. Controle da dor no pós-operatório. Rev Esc Enferm USP. 2001;35(2):180–3.
Polomano RC, Dunwoody CJ, Krenzischek D a, Rathmell JP. Perspective on pain management in the 21st century. Pain Manag Nurs. 2008;9(1 Suppl):S3–10.
Argoff C. Review mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27(10):2019–31.
Ashburn MA. Future challenges in anesthesia-based acute postoperative pain management. ASA Refresher Courses Anesthesiol. 1999;27(1):1–12.
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40.
Harmatz A. Local anesthetics: uses and toxicities. Surg Clin. 2009;89:587–98. https://doi.org/10.1016/j.suc.2009.03.008.
Masters DB, Berde CB, Dutta S, Turek T, Langer R. Sustained local anesthetic release from bioerodible polymer matrices: a potential method for prolonged regional anesthesia. Pharm Res. 1993;10:1527–32.
Exparel. [Bula]. Nova Jersey: Pacira Pharmaceuticals; 2011.
Butz DR, Shenaq DS, Rundell VLM, Kepler B, Liederbach E, Thiel J, et al. Postoperative pain and length of stay lowered by use of exparel in immediate, implant-based breast reconstruction. Plast Reconstr Surg - Glob Open [Internet]. 2015;3(5):e391. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01720096-201505000-00003
Ilfeld BM, Viscusi ER, Hadzic A, Minkowitz HS, Morren MD, Lookabaugh J, et al. Safety and side effect profile of liposome bupivacaine (exparel) in peripheral nerve blocks. Reg Anesth Pain Med [Internet]. 2015;40(5):572–82. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00115550-201509000-00018
Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty [Internet]. 2015;30(2):325–9. Available from: https://doi.org/10.1016/j.arth.2014.09.004
Novabupi. [Bula]. São Paulo: CRISTALIA; 2013.
Foster RH, Markham A. Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs [Internet]. 2000 Mar [cited 2014 Oct 6];59(3):551–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10776835.
Simonetti MPB, Valineti EA, Ferreira FMC. Avaliação Da Atividade Anestésica Local Da S(−)Bupivacaína: Estudo Experimental In Vivo No Nervo Ciático De Rato. Rev Bras Anestesiol. 1997;47(5):424–34.
Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release [Internet]. 2000;67(2–3):357–68. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168365900002315
Simonetti MPB, Batista RA, Ferreira FMC, Estereoisomeria A. Interface Da Tecnologia Industrial De Medicamentos E Da Racionalização Terapêutica. Rev Bras Anestesiol. 1998;48(5):390–9.
Bergamaschi F, Balle VR, Gomes MEW, Machado SB, Mendes FF. Levobupivacaína Versus Bupivacaína emAnestesia Peridural para Cesarianas. Estudo Comparativo Rev Bras Anestesiol. 2005;55(6):606–13.
DA Silva GHR, Ribeiro LN, Mitsutake H, Guilherme VA, Castro SR, Poppi RJ, et al. Optimised NLC: a nanotechnological approach to improve the anaesthetic effect of bupivacaine. Int J Pharm. 2017;529:253–63. https://doi.org/10.1026/j.ijpharm.2017.06.066.
Grillo R, de Melo NFS, de Araújo DR, de Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18(9):688–99.
Cuming RS, Abarca EM, Duran S, Wooldridge AA, Stewart AJ, Ravis W, et al. Development of a sustained-release voriconazole-containing thermogel for subconjunctival injection in horses. Invest Ophthalmol Vis Sci. 2017;58:2746–54. https://doi.org/10.1167/iovs.16-20899.
Kondiah PJ, Choonara YE, Kondiah PPD, Kumar P, Marimuthu T, du Toit LC, et al. Development of an injectable pseudo-bone thermo-gel for application in small bone fractures. Int J Pharm. 2017;520:39–48. https://doi.org/10.1016/j.ijpharm.2017.01.039.
Din UD, Kim F, Choi DW, Thapa RK, Mustapha O, Kim DS, et al. Irinotecan-loaded double-reversible thermogel with improved antitumor efficacy without initial burst effect and toxicity for intramuscular administration. Acta Biomater. 2017;54:239–48. https://doi.org/10.1016/j.actbio.2017.03.007.
Chen Y, Li Y, Shen W, Li K, Yu L, Chen Q, Ding J. Controlled release of liraglutide using thermogelling polymers in treatment of diabetes. Sci Rep. 2016; 31593 doi: https://doi.org/10.1038/srep31593.
Schmolka IR. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res [Internet]. 1972;6(6):571–82. Available from: http://doi.wiley.com/10.1002/jbm.820060609
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res [Internet]. 2006 Dec [cited 2014 Nov 7]; 23(12):2709–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17096184
Charrueau C, Tuleu C, Astre V, Grossiord J-L, Chaumeil J-C. Poloxamer 407 as a thermogelling and adhesive polymer for rectal administration of short-chain fatty acids. Drug Dev Ind Pharm [Internet]. 2001;27(4):351–7. Available from: www.dekker.com
Wang X, Zhao X, Lin T, Guo H. Thermo-sensitive hydrogel for preventing bowel injury in percutaneous renal radiofrequency ablation. Int Urol Nephrol [Internet]. 2016;48(10):1593–600. Available from: http://link.springer.com/10.1007/s11255-016-1349-1
Jiang Y, Meng X, Wu Z, Qi X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr Polym [Internet]. 2016;144:245–53. https://doi.org/10.1016/j.carbpol.2016.02.059.
Mazia RS, De Araújo Pereira RR, De Francisco LMB, Natali MRM, Dias Filho BP, Nakamura CV, et al. Formulation and evaluation of a mucoadhesive thermoresponsive system containing Brazilian green propolis for the treatment of lesions caused by herpes simplex type i. J Pharm Sci. 2016;105(1):113–21.
Svirskis D, Chandramouli K, Bhusal P, Wu Z, Alphonso J, Chow J, et al. Injectable thermosensitive gelling delivery system for the sustained release of lidocaine. Ther Deliv [Internet]. 2016;7(6):359–68. Available from: http://www.future-science.com/doi/10.4155/tde.15.92
Ur-Rehman T, Tavelin S, Gröbner G. Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int J Pharm [Internet]. 2011;409(1–2):19–29. https://doi.org/10.1016/j.ijpharm.2011.02.017.
Lin J-Y, Lai P-L, Lin Y-K, Peng S, Lee L-Y, Chen C-N, et al. A poloxamer-polypeptide thermosensitive hydrogel as cell scaffold and sustained release depot. Polym Chem. 2016;7:2976–85.
De Souza Ferreira SB, Da Silva JB, Borghi-Pangoni FB, Junqueira MV, Bruschi ML. Linear correlation between rheological, mechanical and mucoadhesive properties of polycarbophil polymer blends for biomedical applications. J Mech Behav Biomed Mater [Internet]. 2017;68(2016):265–75. https://doi.org/10.1016/j.jmbbm.2017.02.016.
Borghi-Pangoni FB, Junqueira MV, De Souza Ferreira SB, Silva LL, Rabello BR, Caetano W, et al. Screening and in vitro evaluation of mucoadhesive thermoresponsive system containing methylene blue for local photodynamic therapy of colorectal cancer. Pharm Res. 2016;33(3):776–91.
Rençber S, Karavana SY, Şenyiğit ZA, Eraç B, Limoncu MH, Baloğlu E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm Dev Technol [Internet]. 2016;7450(April):1–11. Available from: http://www.tandfonline.com/doi/full/10.3109/10837450.2016.1163385
Radivojša M, Grabnar I, Grabnar PA. Thermoreversible in situ gelling poloxamer-based systems with chitosan nanocomplexes for prolonged subcutaneous delivery of heparin: design and in vitro evaluation. Eur J Pharm Sci [Internet]. 2013;50(1):93–101. https://doi.org/10.1016/j.ejps.2013.03.002.
Huang W, Zhang N, Hua H, Liu T, Tang Y, Fu L, et al. Preparation, pharmacokinetics and pharmacodynamics of ophthalmic thermosensitive in situ hydrogel of betaxolol hydrochloride. Biomed Pharmacother [Internet]. 2016;83:107–13. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0753332216305686
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Da Silva JA, De Santana DP, Bedor DGC, Borba VFDC, Lira AAM, Egito ESTD. Estudo de liberação e permeaçãoinvitro do diclofenaco de dietilamônio em microemulsão gel-like. Quim Nova. 2009;32(6):1389–93.
MONTEIRO M, GOTARDO MA. Ftalato de di-(2-etilexila) (DEHP) em bolsas de PVC para soluções parenterais de grandes volumes. Rev Ciências Farm Básica e Apl. 2005;26(1):9–18.
Arbelaez-Camargo D, Suñé-Negre JM, Roig-Carreras M, García-Montoya E, Pérez-Lozano P, Miñarro-Carmona M, et al. Preformulation and characterization of a lidocaine hydrochloride and dexamethasone sodium phosphate thermo-reversible and bioadhesive long-acting gel for intraperitoneal administration. Int J Pharm. 2016;498(1–2):142–52.
Barse R, Kokare C, Tagalpallewar A. Influence of hydroxypropylmethylcellulose and poloxamer composite on developed ophthalmic in situ gel: ex vivo and in vivo characterization. J Drug Deliv Sci Technol [Internet]. 2016;33:66–74. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1773224716301034
Gonjari ID, Karmarkar AB, Khade TS, Hosmani AH, Navale RB. Use of factorial design in formulation and evaluation of ophthalmic gels of gatifloxacin: comparison of different mucoadhesive polymers. Drug Discov Ther. 2010;4(6):423–34.
Kabanov AV, Batrakova EV, Valery YA. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212.
Boffito M, Gioffredi E, Chiono V, Calzone S, Ranzato E, Martinotti S, et al. Novel polyurethane-based thermosensitive hydrogels as drug release and tissue engineering platforms: design and in vitro characterization. Polym Int. 2016;65(7):756–69.
Chung Y-M, Simmons KL, Gutowska A, Jeong B. Sol-gel transition temperature of PLGA-g-PEG aqueous solutions. Biomacromolecules [Internet]. 2002;3(3):511–6. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12005522&retmode=ref&cmd=prlinks%5Cnpapers2://publication/uuid/0A979C20-72A7-4487-877F-C711E265EC65
Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103(3):609–24.
Deka D, Deb P, Dash S. Formulation development and evaluation of thermosensitive oral gel containing doxycycline hyclate. WORLD J Pharm Pharm Sci. 2016;5(6):1346–57.
Shastri DH, Dodiya HD, Shelat P, Bhanupriy AK. Formulation development and evaluation of a gastroretentive in situ oral gel of Cefuroxime Axetil. J Young Pharm [Internet]. 2016;8(4):324–9. Available from: http://www.jyoungpharm.org/article/891
Bruschi ML, Jones DS, Panzeri H, Gremião MPD, de Freitas O, Lara EHG. Semisolid systems containing propolis for the treatment of periodontal disease: in vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J Pharm Sci [Internet]. 2006;96(8):2074–89. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022354916323243
Junqueira MV, Borghi-Pangoni FB, Ferreira SB, Bruschi ML. Evaluation of the methylene blue addition in binary polymeric systems composed by poloxamer 407 and Carbopol 934P using quality by design: rheological, textural, and mucoadhesive analysis. Drug Dev Ind Pharm [Internet]. 2016;9045(May):1–41. Available from: http://www.tandfonline.com/doi/full/10.1080/03639045.2016.1188111
Li Y, Rodrigues J, Tomás H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev [Internet]. 2012;41(6):2193–221. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22116474
Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm [Internet]. 2008;68(1):34–45. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0939641107002470
Yu L, Li K, Liu X, Chen C, Bao Y, Ci T, et al. In vitro and in vivo evaluation of a once-weekly formulation of an antidiabetic peptide drug exenatide in an injectable thermogel. J Pharm Sci. 2013;102(11):4140–9.
Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 2002;85:73–81. https://doi.org/10.1016/S0168-3659(02)00273-0.
Huang L, Shen M, Li R, Zhang X, Sun Y, Gao P, et al. Thermo-sensitive composite hydrogels based on poloxamer 407 and alginate and their therapeutic effect in embolization in rabbit VX2 liver tumors. Oncotarget [Internet]. 2016;1–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27602579
Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. https://doi.org/10.1016/j.ejpb.2004.03.019.
Balzus B, Colombo M, Sahle FF, Zoubari G, Staufenbiel S, Bodmeier R. Comparison of different in vitro release methods used to investigate nanocarriers intended for dermal application. Int J Pharm [Internet]. 2016;513(1–2):247–54. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0378517316308638
de Araújo Pereira RR, Ribeiro Godoy JS, Stivalet Svidzinski TI, Bruschi ML. Preparation and characterization of mucoadhesive thermoresponsive systems containing propolis for the treatment of vulvovaginal candidiasis. J Pharm Sci. 2013;102(4):1222–34.
Qi X, Qin X, Yang R, Qin J, Li W, Luan K, et al. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. J Pharm Sci [Internet]. 2016;105(1):122–30. https://doi.org/10.1016/j.xphs.2015.11.019.
Panapisal V, Charoensri S, Tantituvanont A. Formulation of microemulsion systems for dermal delivery of silymarin. AAPS PharmSciTech [Internet]. 2012 Jun [cited 2014 Sep 16];13(2):389–99. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3364378&tool=pmcentrez&rendertype=abstract.
Akkari ACS, Papini JZB, Garcia GK, Franco MKKD, Cavalcanti LP, Gasperini A, et al. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesia: Physico-chemical characterization and pharmacological evaluation. Mater Sci Eng C [Internet]. 2016;68:299–307. https://doi.org/10.1016/j.msec.2016.05.088.
Ricci EJ, Lunardi LO, Nanclares DMA, Marchetti JM. Sustained release of lidocaine from Poloxamer 407 gels. Int J Pharm. 2005;288(2):235–44.
Suh H, Jun HW. Physicochemical and release studies of naproxen in poloxamer gels. Int J Pharm [Internet]. 1996;129(1–2):13–20. Available from: http://linkinghub.elsevier.com/retrieve/pii/0378517395042105
Anderson BC, Pandit NK, Mallapragada SK. Understanding drug release from poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) gels. J Control Release. 2001;70(1–2):157–67.
Paavola A, Yliruusi J, Kajimoto Y, Kalso E, Wahlstrom T, Rosenberg P. Controlled release of lidocaine from injectable gels and efficacy in rat sciatic nerve block. Pharm Res. 1995;12:1997–2002.
Cavallari C, Brigidi P, Fini A. Ex-vivo and in-vitro assessment of mucoadhesive patches containing the gel-forming polysaccharide psyllium for buccal delivery of chlorhexidine base. Int J Pharm [Internet]. 2015;496(2):593–600. https://doi.org/10.1016/j.ijpharm.2015.10.077.
Acknowledgements
The authors would like to thank Cristália Prod. Quim. Farm. LTDA for kindly providing Novapubi®, NUDFAC/Farmácia Escola Carlos Drummond de Andrade/UFPE, and Centro de Tecnologias Estratégicas do Nordeste (CETENE).
Funding
This study received a grant from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
These experiments on rats were approved by the Ethical Committee of the Federal University of Pernambuco (Registration no. 23076.007923/2017-61) and performed in automated Franz diffusion cells (Vision® Microette automated diffusion test system, Chatsworth, USA).
Rights and permissions
About this article
Cite this article
de Lima, E.N., de Andrade, A.R.B., Leal, L.B. et al. Levobupivacaine Thermogel for Long-acting Analgesia. AAPS PharmSciTech 19, 2533–2542 (2018). https://doi.org/10.1208/s12249-018-1083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1083-3