Abstract
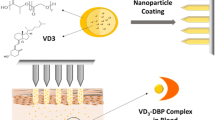
Vitamin D3 supplementation is important to prevent and treat hypovitaminosis that is a worldwide public health issue. Most types of supplementation are by oral route or fortification foods. The alternative route must be investigated, as transdermal route, for people with fat malabsorption or other diseases that impair the absorption of vitamin D3. This study focused on verifying the feasibleness of vitamin D3 skin retention and permeation with the presence of chemical penetration enhancers (soybean lecithin, isopropyl palmitate, propylene glycol, ethoxydiglycol, and cereal alcohol) at different pharmaceutical forms (gel and cream) through a human skin. The integrity of skin was evaluated by transepidermal water loss (TEWL) during the skin retention and permeation test. The combination of chemical penetration enhancers presented in cream did not compromise the skin, different from the gel that association of cereal alcohol and propylene glycol compromised the skin in 24 h. Gel formulation showed vitamin D3 detection at stratum corneum in 4 h and at epidermis and dermis in 24 h. Vitamin D3 demonstrated an affinity with the vehicle in the cream formulation and was detected at the skin surface. No active was found at receptor fluid for both formulations. In conclusion, the vitamin D3 did not indicate feasibleness for transdermal use probably due to its physical-chemical characteristics such as high lipophilicity since it was not permeated through a human skin. Nevertheless, the transdermal route should be continuously investigated with less lipophilic derivates of vitamin D3 and with different combination of penetration enhancers.






Similar content being viewed by others
References
Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–45.
Bishai D, Nalubola R. The history of food fortification in the United States: its relevance for current fortification efforts in developing countries. Econ Dev Cult Chang. 2002;51(1):37–53.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S.
Gilaberte Y, Aguilera J, Carrascosa JM, Figueroa FL, Romaní de Gabriel J, Nagore E. Vitamin D: evidence and controversies. Actas Dermosifiliogr. 2011;102(8):572–88.
Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1–2):297–300.
Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med. 2010;235(9):1034–45.
Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115–24.
Alsaqr A, Rasoully M, Musteata FM. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech. 2015;16(4):963–72.
Sadat-Ali M, Bubshait DA, Al-Turki HA, Al-Dakheel DA, Al-Olayani WS. Topical delivery of vitamin D3: a randomized controlled pilot study. Int J Biomed Sci. 2014;10(1):21–4.
Ramezanli T, Kilfoyle BE, Zhang Z, Michniak-Kohn BB. Polymeric nanospheres for topical delivery of vitamin D3. Int J Pharm. 2017;516(1–2):196–203.
Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, et al. Skin barrier and transdermal drug delivery. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. New York: Elsevier Health Sciences; 2012. p. 2065–73.
Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788(11):2362–73.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37.
Guo H, Liu Z, Li J, Nie S, Pan W. Effects of isopropyl palmitate on the skin permeation of drugs. Biol Pharm Bull. 2006;29(11):2324–6.
Ghebre-sellassie I, Martin C, editors. Pharmaceutical Extrusion Technology: Drugs and the pharmaceutical sciences. New York: Inform Healthcare. p. 2007.
Batistuzzo JAO, Itaya M, Eto Y. Formulário Médico-Farmacéutico. 4th ed. São Paulo: Pharmabooks Editora; 2011.
Bilodeau L, Dufresne G, Deeks J, Clément G, Bertrand J, Turcotte S, et al. A Determination of vitamin D3 and 25-hydroxyvitamin D3 in foodstuffs by HPLC UV-DAD and LC–MS/MS. J Food Compos Anal. 2011;24:441–8.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization. 2005. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed 27 Apr 2016.
European Medicines Agency. Guideline on quality of transdermal patches Guideline on quality of transdermal patches Table of contents, London, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/ Scientific_guideline/2014/12/WC500179071.pdf. Accessed 15 Aug 2016.
Leite-Silva VR, Almeida MM, Fradin A, Grice JE, Roberts MS. Delivery of drugs applied topically to the skin. Expert Rev Dermatol. 2012;7(4):383–97.
Armelini AIPV. Evaluation of the in vitro dermal absorption profile of daidzein in a cosmetic nanoemulsion: an approach focused on the safety assessment [Dissertation].Ribeirão Preto: University of São Paulo; 2015.
Directorate E, Meeting J, THE OF, Committee C, working THE, on P, et al. Guideance notes on dermal absorption. Series on testing and assessment. No. 156. Paris: Organization for Economic co-operation and Development; 2011. p. 1–72.
Gioia F, Celleno L. The dynamics of transepidermal water loss (TEWL) from hydrated skin. Skin Res Technol. 2002;8(3):178–86.
Guth K, Schäfer-Korting M, Fabian E, Landsiedel R, van Ravenzwaay B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol in Vitro. 2015;29(1):113–23.
Franz TJ. Percutaneous absorption. On the relevance of in vitro data. J Invest Dermatol. 1975;64(30):190–5.
World Health Organization. Dermal absorption: Environmental health criteria 235. 2006. http://www.inchem.org/documents/ehc/ehc/ehc235.pdf. Accessed 15 Aug 2016.
U.S. Pharmacopeial Convention. U. S. Pharmacopeia National Formulary, USP 39 NF 34, vol. 1. Baltimore: United Book Pres; 2016. p. 1869–81.
Box GEP, Hunter JS, Hunter WG, editors. Statistics for experiments: Design, innovation and discovery. 2nd ed. New Jersey: Wiley-Interscience; 2005.
patchmd.com [Internet]. Las Vegas: Patch MD; c2017 [cited 2017 Nov 06]. Available from: https://www.patchmd.com/Vitamin-D3-Topical-Patch.html.
primal-d.com [Internet]. Louisville: Primal-D; c2017 [cited 2017 Nov 06]. Available from: http://primal-d.com/.
betteryou.com [Internet]. Barnsley: Better You; c2017 [cited 2017 Nov 06]. Available from: https://betteryou.com/vitamin-d-oral-sprays.
amazon.com [Internet]. Seattle: Amazon; c2017 [cited 2017 Nov 06]. Available from: https://www.amazon.com/AnuMed-Vitamin-Cream-000-Ounces/dp/B00454A1HE?th=1.
Saunders M, Pugh W. Use of ethanol in receptor phase—a lucky escape? In: Brain KR, Walters KA, editors. Perspectives in percutaneous penetration. Abstracts of presentations at the eighth international perspectives in percutaneous penetration conference held in Antibes-Juan-les-Prins, vol. 8a. Cardiff: STS Publishing; 2002. p. 21.
De Paépe K, Hachem JP, Vanpee E, Goossens A, Germaux MA, Lachapelle JM, et al. Beneficial effects of a skin tolerance-tested moisturizing cream on the barrier function in experimentally elicited irrita-tion and allergic contact dermatitis. Contact Derm. 2001;44(6):337–43.
Loftsson T, Hreinsdôttir D. Determination of aqueous solubility by heating and equilibration: a technical note. AAPS PharmSciTech. 2006;7(1):E29–32.
U.S. Food and Drug Administration. Vectical (calcitriol) ointment 3 mcg/g: for topical use only, Texas. 2009. https://www.accessdata.fda.gov/ drugsatfda_docs/label/2009/022087lbl.pdf. Accessed 25 Sept 2017.
Acknowledgments
We would like to acknowledge the UNIFESP for the partnership and USP for all academic support. This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—no. 159265/2015-0 (GMDC)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Angelo Costa, G.M., Sales de Oliveira Pinto, C.A., Rodrigues Leite-Silva, V. et al. Is Vitamin D3 Transdermal Formulation Feasible? An Ex Vivo Skin Retention and Permeation. AAPS PharmSciTech 19, 2418–2425 (2018). https://doi.org/10.1208/s12249-018-1065-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1065-5