Abstract
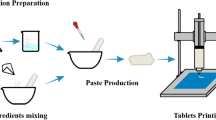
3D printing evolved as a promising technique to improve individualization of drug therapy. In particular, when printing sustained release solid dosage forms, as for instance implants, inserts, and also tablets, estimation of the drug release profile in vivo is necessary. In most cases, corresponding analyses cannot be performed at hospital or community pharmacies. Therefore, the present study aimed to develop a sustained release drug delivery system produced via 3D printing, which allows dose adaption and estimation of drug release at the same time. Filaments as feedstock for the printer were produced via hot-melt extrusion and consisted of Eudragit® RL as sustained release polymer, 30% theophylline as model active pharmaceutical ingredient, and stearic acid as solid plasticizer. Assuming that the surface/mass ratio was constant, network structures of different densities were printed as novel solid dosage form. Their weight (263 to 668 mg), thereby their dose, and surface area, determined using X-ray microcomputed tomography, showed a linear correlation with the fill density. The specific surface area of the network hardly varied with changing fill density. Dissolution studies showed a slower drug release for dosage forms with a denser network. Higuchi’s model was used for prediction of drug release and showed limited applicability due to different release kinetics for different fill densities. However, using linear interpolation for the prediction resulted in good RMSEP values between 1.4 and 3.7%. These findings might be useful to enable customized production of sustained release solid dosage forms via 3D printing in hospital and community pharmacies in the future.









Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- CT:
-
Computed tomography
- DSC:
-
Differential scanning calorimetry
- EC:
-
Ethyl cellulose
- FDM:
-
Fused deposition modeling
- HME:
-
Hot-melt extrusion
- HPMC:
-
Hydroxypropyl methylcellulose
- RMSEP:
-
Root mean square error of prediction
- SSA:
-
Specific surface area
2. References
Schlender JF, Meyer M, Thelen K, Krauss M, Willmann S, Eissing T, et al. Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet. 2016;55(12):1573–89. https://doi.org/10.1007/s40262-016-0422-3.
Klotz U. The elderly—a challenge for appropriate drug treatment. Eur J Clin Pharmacol. 2008;64(3):225–6. https://doi.org/10.1007/s00228-007-0410-5.
Breitkreutz J, Boos J. Paediatric and geriatric drug delivery. Expert Opin Drug Deliv. 2007;4(1):37–45. https://doi.org/10.1517/17425247.4.1.37.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology - drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. https://doi.org/10.1056/NEJMra035092.
Wening K, Breitkreutz J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int J Pharm. 2011;404(1–2):1–9. https://doi.org/10.1016/j.ijpharm.2010.11.001.
Wening K, Breitkreutz J. Novel delivery device for monolithical solid oral dosage forms for personalized medicine. Int J Pharm. 2010;395(1–2):174–81. https://doi.org/10.1016/j.ijpharm.2010.05.036.
Laukamp EJ, Knop K, Thommes M, Breitkreutz J. Micropellet-loaded rods with dose-independent sustained release properties for individual dosing via the solid dosage pen. Int J Pharm. 2016;499(1–2):271–9. https://doi.org/10.1016/j.ijpharm.2016.01.001.
Giannatsis J, Dedoussis V. Additive fabrication technologies applied to medicine and health care: a review. Int J Adv Manuf Technol. 2009;40(1–2):116–27. https://doi.org/10.1007/s00170-007-1308-1.
Tiwari RV, Patil H, Repka MA. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin Drug Deliv. 2016;13(3):451–64. https://doi.org/10.1517/17425247.2016.1126246.
Pietrzak K, Isreb A, Alhnan MA. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. 2015;96:380–7. https://doi.org/10.1016/j.ejpb.2015.07.027.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7. https://doi.org/10.1016/j.ejps.2014.11.009.
Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–8. https://doi.org/10.1016/j.jconrel.2016.05.034.
Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63. https://doi.org/10.1016/j.ijpharm.2015.04.069.
Masood SH, Singh S (eds) Design of micro-features in polymeric drug delivery devices using FDM. ASME International Mechanical Engineering Congress and Exposition. Proceedings (IMECE). 2007.
Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–97. https://doi.org/10.1016/j.ijpharm.2016.12.049.
Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509(1–2):255–63. https://doi.org/10.1016/j.ijpharm.2016.05.036.
Zhang J, Yang W, Vo AQ, Feng X, Ye X, Kim DW, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. https://doi.org/10.1016/j.carbpol.2017.08.058.
Kavanagh N, Corrigan OI. Swelling and erosion properties of hydroxypropylmethylcellulose (Hypromellose) matrices—influence of agitation rate and dissolution medium composition. Int J Pharm. 2004;279(1–2):141–52. https://doi.org/10.1016/j.ijpharm.2004.04.016.
Sun Y, Soh S. Printing tablets with fully customizable release profiles for personalized medicine. Adv Mater. 2015;27(47):7847–53. https://doi.org/10.1002/adma.201504122.
Goyanes A, Fina F, Martorana A, Sedough D, Gaisford S, Basit AW. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int J Pharm. 2017;527(1–2):21–30. https://doi.org/10.1016/j.ijpharm.2017.05.021.
Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA. Fabricating a Shell-Core delayed release tablet using dual FDM 3D printing for patient-Centred therapy. Pharm Res. 2017;34(2):427–37. https://doi.org/10.1007/s11095-016-2073-3.
Kempin W, Franz C, Koster L-C, Schneider F, Bogdahn M, Weitschies W, et al. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur J Pharm Biopharm. 2017;115:84–93. https://doi.org/10.1016/j.ejpb.2017.02.014.
Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261:207–15. https://doi.org/10.1016/j.jconrel.2017.06.025.
Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476(1):88–92. https://doi.org/10.1016/j.ijpharm.2014.09.044.
Li Q, Guan X, Cui M, Zhu Z, Chen K, Wen H, et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int J Pharm. 2018;535(1):325–32. https://doi.org/10.1016/j.ijpharm.2017.10.037.
Yang Y, Wang H, Li H, Ou Z, Yang G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur J Pharm Sci. 2018;115:11–8. https://doi.org/10.1016/j.ejps.2018.01.005.
Thakral S, Thakral NK, Majumdar DK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–49. https://doi.org/10.1517/17425247.2013.736962.
Quinten T, Andrews GP, De Beer T, Saerens L, Bouquet W, Jones DS, et al. Preparation and evaluation of sustained-release matrix tablets based on metoprolol and an acrylic carrier using injection moulding. AAPS PharmSciTech. 2012;13(4):1197–211. https://doi.org/10.1208/s12249-012-9848-6.
Korte C, Quodbach J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm Dev Technol. 2018:1–11. https://doi.org/10.1080/10837450.2018.1433208.
European Directorate for the Quality of Medicines & HealthCare. Monograph 51701: Recommendations on dissolution testing. European Pharmacopoeia 9.0 ed. Strasbourg: European Directorate for the Quality of Medicines & HealthCare; 2017. p. 761–763.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35. https://doi.org/10.1016/0378-5173(83)90064-9.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(12):1145–9. https://doi.org/10.1002/jps.2600521210.
Barimani S, Kleinebudde P. Monitoring of tablet coating processes with colored coatings. Talanta. 2018;178:686–97. https://doi.org/10.1016/j.talanta.2017.10.008.
Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50(10):874–5. https://doi.org/10.1002/jps.2600501018.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33. https://doi.org/10.1016/S0928-0987(01)00095-1.
Siepmann J, Siepmann F. Modeling of diffusion controlled drug delivery. J Control Release. 2012;161(2):351–62. https://doi.org/10.1016/j.jconrel.2011.10.006.
Acknowledgments
The authors would like to thank Dorothee Eikeler for her support in the content determination of the matrices, Stefan Stich for preparing in-house-built sinkers, and Karin Matthée for conduction of DSC measurements. Further, Raphael Wiedey is acknowledged for his support in the conduction and evaluation of measurements using X-ray microcomputed tomography.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Niklas Sandler and Jukka Rantanen
Rights and permissions
About this article
Cite this article
Korte, C., Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 19, 3333–3342 (2018). https://doi.org/10.1208/s12249-018-1017-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1017-0