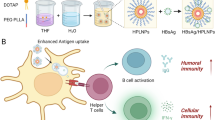
Abstract
Traditional parenteral recombinant hepatitis B virus (HBV) vaccines have effectively reduced the disease burden despite being able to induce seroprotective antibody titers in 5–10% vaccinated individuals (non-responders). Moreover, an estimated 340 million chronic HBV cases are in need of treatment. Development of safe, stable, and more effective hepatitis B vaccine formulation would address these challenges. Recombinant hepatitis B surface antigen (rHBsAg) entrapped solid fat nanoemulsions (SFNs) containing monophosphoryl lipid A (MPLA) that was prepared and optimized by quality by design (QbD) using response surface methodology (RSM), i.e., central composite design (CCD). Its immune potential was evaluated with preset immunization protocol in a murine model. Dose escalation study revealed that formulation containing 1 μg of rHBsAg entrapped SFNs with MPLA-induced significant higher humoral, and cellular response compared to the marketed vaccine (Genvac B) administered intramuscularly. SFNs with nanometric morphology and structural similarity with chylomicrons assist in improved uptake and processing to lymphatics. Moreover, the presence of an immunogenic component in its structure further augments delivery of rHBsAg to immune cells with induction of danger signals. This multi-adjuvant based approach explores new prospect for the dose sparing. Improved cellular immune response induced by this vaccine formulation suggests that it could be tested as an immunotherapeutic vaccine as well.









Similar content being viewed by others
References
Dormitzer PR, Ulmer JB, Rappuoli R. Structure-based antigen design: a strategy for next generation vaccines. Trends Biotechnol. 2008;26(12):659–67. https://doi.org/10.1016/j.tibtech.2008.08.002.
Brito LA, Malyala P, O’Hagan DT. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol. 2013;25(2):130–45. https://doi.org/10.1016/j.smim.2013.05.007.
Vanlandschoot P, Leroux-Roels G. Hepatitis B vaccines: accomplishments, shortcomings, and future developments. S Afr J Epidemiol Infect. 2008;23(1):33–7. https://doi.org/10.1080/10158782.2008.11441298.
Zehrung D, Jarrahian C, Wales A. Intradermal delivery for vaccine dose sparing. Vaccine. 2013;31(34):3392–5. https://doi.org/10.1016/j.vaccine.2012.11.021.
Manesis EK, Cameron CH, Gregoriadis G, Hepatitis B. Surface antigen-containing liposomes enhance humoral and cell-mediated immunity to the antigen. FEBS Lett. 1979;102(1):107–11. https://doi.org/10.1016/0014-5793(79)80939-4.
Damme PV, Oosterhuis-Kafeja F, Wielen MV, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27(3):454–9. https://doi.org/10.1016/j.vaccine.2008.10.077.
Anselem S, Lowell GH, Aviv H, Friedman D. Solid fat nanoemulsions as vaccine delivery vehicles. United States Patent no. 5,716,637, 10 February 1998
Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int J Pharm. 2009;380(1-2):181–8. https://doi.org/10.1016/j.ijpharm.2009.06.026.
Kaviratna AS, Banerjee R. The effect of acids on dipalmitoyl phosphatidylcholine (DPPC) monolayers and liposomes. Colloids Surf A Physicochem Eng Asp. 2009;345(1-3):155–62. https://doi.org/10.1016/j.colsurfa.2009.04.051.
Mady MM, Darwish MM. Effect of chitosan coating on the characteristics of DPPC liposomes. J Adv Res. 2010;1(3):187–91. https://doi.org/10.1016/j.jare.2010.05.008.
Chong CSW, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS, et al. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticles vaccine delivery. J Control Release. 2005;102(1):85–99. https://doi.org/10.1016/j.jconrel.2004.09.014.
Lionberger RA, Lee SL, Lee LM, Raw A, Yu LX. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–73. https://doi.org/10.1208/s12248-008-9026-7.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83. https://doi.org/10.1208/s12248-014-9598-3.
Riley BS, Li X. Quality by design and process analytical Technology for Sterile Products—Where are we now? AAPS PharmSciTech. 2011;12(1):114–8. https://doi.org/10.1208/s12249-010-9566-x.
Cook J, Cruañes MT, Gupta M, Riley S, Crison J. Quality-by-design: are we there yet? AAPS PharmSciTech. 2014;15(1):140–8. https://doi.org/10.1208/s12249-013-0043-1.
Sangshetti JN, Deshpande M, Zaheer Z, Shinde DB, Arote R. Quality by design approach: Regulatory need. Arab J Chem. 2017;10(2):S3412–25. https://doi.org/10.1016/j.arabjc.2014.01.025.
Minz S, Kaurav M, Sahu KK, Mandal V, Pandey RS. Development and validation of TLC-densitometric method for determination of lipid A adjuvant as a bulk and in solid fat nanoemulsions. Biomed Chromatogr. 2015;29(10):1473–9. https://doi.org/10.1002/bmc.3444.
Gonzalez-Mira E, Egea M, Souto E, Calpena A, García M. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2011;22(4):045101. https://doi.org/10.1088/0957-4484/22/4/045101.
Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, et al. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47(2):497–05. https://doi.org/10.1016/j.ejps.2012.07.006.
Pradhan M, Singh D, Singh MR. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem Phys Lipids. 2015 Feb;186:9–16. https://doi.org/10.1016/j.chemphyslip.2014.11.004.
Zhang JA, Anyarambhatla G, Ma L, Ugwu S, Xuan T, Sardone T, et al. Development and characterization of a novel Cremophor® EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm. 2005;59(1):177–87. https://doi.org/10.1016/j.ejpb.2004.06.009.
Maheshwari C, Pandey RS, Chaurasiya A, Kumar A, Selvam DT, Prasad GBKS, et al. Non-ionic surfactant vesicles mediated transcutaneous immunization against hepatitis B. Int Immunopharmacol. 2011;1:1516–22.
Morton RE, Evans TA. Modification of the Bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal Biochem. 1992;204(2):332–4. https://doi.org/10.1016/0003-2697(92)90248-6.
Gupta PN, Mahor S, Rawat A, Khatri K, Goyal A, Vyas SP. Lectin anchored stabilized biodegradable nanoparticles for oral immunization, development and in vitro evaluation. Int J Pharm. 2006;318(1-2):163–73. https://doi.org/10.1016/j.ijpharm.2006.03.017.
Pandey RS, Babbar AK, Kaul A, Mishra AK, Dixit VK. Evaluation of ISCOM matrices clearance from rabbit nasal cavity by gamma scintigraphy. Int J Pharm. 2010;398(1-2):231–6. https://doi.org/10.1016/j.ijpharm.2010.07.051.
Pandey RS, Dixit VK. Evaluation of ISCOM vaccines for mucosal immunization against hepatitis B. J Drug Target. 2010;18(4):282–91. https://doi.org/10.3109/10611860903450015.
Pandey RS, Sahu S, Sudheesh MS, Madan J, Kumar M, Dixit VK. Carbohydrate modified ultrafine ceramic nanoparticles for allergen immunotherapy. Int Immunopharmacol. 2011;11(8):925–31. https://doi.org/10.1016/j.intimp.2011.02.004.
Greiner VJ, Ronzon F, Larquet E, Desbat B, Esteves C, Bonvin J, et al. The structure of HBsAg particles is not modified upon their adsorption on aluminium hydroxide gel. Vaccine. 2012;30(35):5240–55. https://doi.org/10.1016/j.vaccine.2012.05.082.
Pandey RS, Dixit VK. Evaluation of ISCOMs for immunization against hepatitis B. Curr Pharm Biotechnol. 2009;10(7):709–16. https://doi.org/10.2174/138920109789542093.
Chittasupho C, Lirdprapamongkol K, Kewsuwan P, Sarisuta N. Targeted delivery of doxorubicin to A549 lung cancer cells by CXCR4antagonist conjugated PLGA nanoparticles. Eur J Pharm Biopharm. 2014;88(2):529–38. https://doi.org/10.1016/j.ejpb.2014.06.020.
Zhang J, Chen XG, Peng WB, Liu CS. Uptake of oleoyl-chitosan nanoparticles by A549 cells. Nanomedicine. 2008;4(3):208–14. https://doi.org/10.1016/j.nano.2008.03.006.
Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, et al. Vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid a. Biomaterials. 2011;32(16):4052–7. https://doi.org/10.1016/j.biomaterials.2011.02.011.
Mishra D, Dubey V, Asthana A, Saraf DK, Jain NK. Elastic liposomes mediated transcutaneous immunization against hepatitis B. Vaccine. 2006;24(22):4847–55. https://doi.org/10.1016/j.vaccine.2006.03.011.
Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. Int Immunopharmacol. 2008;8(13-14):1773–80. https://doi.org/10.1016/j.intimp.2008.08.013.
Madan J, Kaushik D, Sardana S, Ali A, Sudheesh MS, Pandey RS. Effect of levofloxacin and pefloxacin on humoral immune response elicited by bovine serum albumin docked in gelatin microparticles and nanoparticles. Pharmazie. 2010;65:1–6.
Lason E, Sikora E, Ogonowski J. Influence of process parameters on properties of nanostructured lipid carriers (NLC) formulation. Acta Biochim Pol. 2003;60:773–7.
Greiner VJ, Manin C, Larquet E, Ikhelef N, Gréco F, Naville S, et al. Characterization of the structural modifications accompanying the loss of HBsAg particle immunogenicity. Vaccine. 2014;32(9):1049–54. https://doi.org/10.1016/j.vaccine.2014.01.012.
Hemling ME, Carr SA, Capiau C, Petre J. Structural characterization of recombinant hepatitis B surface antigen protein by mass spectrometry. Biochemistry. 1988;27(2):699–05. https://doi.org/10.1021/bi00402a031.
Peng J, Tong Y, Ying L, Jie X, Ying Z, Yanna H, et al. Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des Devel Ther. 2016;10:911–25.
Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–22. https://doi.org/10.1016/j.biomaterials.2004.07.050.
Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14(11):1568–73. https://doi.org/10.1023/A:1012126301290.
Foster KA, Yazdanian M, Audus KL. Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J Pharm Pharmacol. 2001;53(1):57–66. https://doi.org/10.1211/0022357011775190.
Couvreur P, Puisieux F. Nano- and microparticles for the delivery of polypeptides and proteins. Adv Drug Deliv Rev. 1993;10(2-3):141–62. https://doi.org/10.1016/0169-409X(93)90046-7.
Kanchan K, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28(35):5344–57. https://doi.org/10.1016/j.biomaterials.2007.08.015.
Sanyal G, Shi LA. Review of multiple approaches towards an improved hepatitis B vaccine. Expert Opin Ther Pat. 2009;19(1):59–72. https://doi.org/10.1517/13543770802587226.
Ucisik MH, Sleytr UB, Schuster B. Emulsomes Meet S-layer proteins: an emerging targeted drug delivery system. Curr Pharm Biotech. 2015;16:392–05.
Guldur T, Karabulut AB, Bayraktar N, Kaynar O. Hydrophobic nature of rat lymph chylomicrons. Clini Chem Acta. 2004;342(1-2):161–9. https://doi.org/10.1016/j.cccn.2003.12.018.
Kretschmar M, Amselem S, Zawoznik E, Mosbach K, Dietz A, Hof H, et al. Efficient treatment of murine systemic infection with Candida albicans using amphotericin B incorporated in nanosize range particles (emulsomes). Mycoses. 2001;44(7–8):281–6. https://doi.org/10.1111/j.1439-0507.2001.00654.x.
Gavilanes F, Gomez-gutierrez J, Aracil M, Gonzalez-ros JM, Ferragut JA, Guerrero E, et al. Hepatitis B surface antigen role of lipids in maintaining the structural and antigenic properties of protein components. Biochem J. 1990;265(3):857–64. https://doi.org/10.1042/bj2650857.
Tleugabulova D. Sodium dodecylsulfate polyacrylamide gel electrophoresis of recombinant hepatitis B surface antigen particles. J Chromatogr B Biomed Sci Appl. 1998;707(1-2):267–73. https://doi.org/10.1016/S0378-4347(97)00567-7.
Saraf S, Mishra D, Asthana A, Jain R, Singh S, Jain NK. Lipid microparticles for mucosal immunization against hepatitis B. Vaccine. 2006;24(1):45–56. https://doi.org/10.1016/j.vaccine.2005.07.053.
Chen D, Tyagi A, Carpenter J, Perkins S, Sylvester D, Guy M, et al. Characterization of the freeze sensitivity of a hepatitis B vaccine. Hum Vaccin. 2009;5:6–32.
Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B: Biointerfaces. 2010;75(1):123–32. https://doi.org/10.1016/j.colsurfb.2009.08.022.
Bungener L, Huckriede A, Wilschut J, Daemen T. Delivery of Protein Antigens to the Immune System by Fusion-active Virosomes: A Comparison with Liposomes and ISCOMs. Biosci Rep. 2002;22(2):323–38. https://doi.org/10.1023/A:1020198908574.
Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64(2):393–01. https://doi.org/10.1016/0092-8674(91)90647-H.
Peachman KK, Rao M, Alving CR, Palmer DR, Sun W, Rothwell SW, et al. Macrophages exhibit different intracellular processing pathways for soluble and liposome-encapsulated antigens. Immunobiology. 2005;201:321–33.
Reddy ST, Swartz MA, Hubbell AJ. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27(12):573–9. https://doi.org/10.1016/j.it.2006.10.005.
Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47(1):55–64. https://doi.org/10.1016/S0169-409X(00)00121-6.
Temmerman MD, Rejman J, Demeester J, Irvine DJ, Gander B, Smedt SCD. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today. 2011;16(13-14):569–82. https://doi.org/10.1016/j.drudis.2011.04.006.
Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29(17):3341–55. https://doi.org/10.1016/j.vaccine.2010.08.002.
Vosika GJ, Barr C, Gilbertson D. Phase-I study of intravenous modified lipid A. Cancer Immunol Immunother. 1984;18(2):107–12.
Baldridge JR, Crane RT. Monophosphoryl lipid a (MPL) formulations for the next generation of vaccine. Methods. 1999;19(1):103–7. https://doi.org/10.1006/meth.1999.0834.
Heppner DG, Gordon DM, Gross M, Wellde B, Leitner W, Krzych U, et al. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996;174(2):361–6. https://doi.org/10.1093/infdis/174.2.361.
Steer NJ, Alving CR, Rao M. Modulation of immunoproteasome subunits by liposomal lipid A. Vaccine. 2008;26(23):2849–59. https://doi.org/10.1016/j.vaccine.2008.03.065.
Leuridan E, Damme PV, Hepatitis B. The need for a booster dose. Clin Infect Dis. 2011;53(1):68–75. https://doi.org/10.1093/cid/cir270.
Khajuria A, Gupta A, Malik F, Singh S, Singh J, Gupta BD, et al. A new vaccine adjuvant (BOS 2000) a potent enhancer mixed Th1/Th2 immune responses in mice immunized with HBsAg. Vaccine. 2007;25(23):4586–94. https://doi.org/10.1016/j.vaccine.2007.03.051.
Isaka M, Yasuda Y, Mizokami M, Kozuka S, Taniguchi T, Matano K, et al. Mucsal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine. 2001;19(11-12):1460–6. https://doi.org/10.1016/S0264-410X(00)00348-0.
Warren HS, Chedid LA. Future prospects for vaccine adjuvants. Crit Rev Immunol. 1988;8(2):83–101.
Gupta RK, Rost BE, Relyveld E, Siber G, Powell MF, Newmandand MK, et al. Adjuvant properties of aluminum and calcium compounds. Vaccine design. New York: Plenum press; 1995. p. 229.
Ulrich JT, Myers KR. Monophosphoryl lipid a as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–24. https://doi.org/10.1007/978-1-4615-1823-5_21.
Bramwell VW, Perrie Y. Particulate delivery systems for vaccines: what can we expect? J Pharm Pharmacol. 2006;58(6):717–28. https://doi.org/10.1211/jpp.58.6.0002.
Brunner R, Jensen-Jarolim E, Pali-Schöll I. The ABC of clinical and experimental adjuvants—a brief overview. Immunol Lett. 2010;128(1):29–35. https://doi.org/10.1016/j.imlet.2009.10.005.
Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19:6.
Airhart CL, Rohde HN, Hovde CJ, Bohach GA, Deobald CF, Lee SS, et al. Mimetics are potent adjuvants for an intranasal pneumonic plague vaccine. Vaccine. 2008;26(44):5554–61. https://doi.org/10.1016/j.vaccine.2008.08.007.
Zeng W, Eriksson E, Chua B, Grollo L, Jackson DC. Structural requirement for the agonist activity of the TLR2 ligand Pam2Cys. Amino Acids. 2010;39(2):471–80. https://doi.org/10.1007/s00726-009-0463-0.
Zeng W, Azzopardi K, Hocking D, Wonga CY, Robevsk GA, Tauschek M, et al. A totally synthetic lipopeptide-based self-adjuvanting vaccine induces neutralizing antibodies against heat-stable enterotoxin from enterotoxigenic Escherichia Coli. Vaccine. 2012;30(32):4800–6. https://doi.org/10.1016/j.vaccine.2012.05.017.
Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv. 2004;1(4):405–12. https://doi.org/10.2174/1567201043334597.
O’Hagan DT, Singh M. Microparticles as vaccine adjuvants and deliverysystems. Expert Rev Vaccines. 2003;2(2):269–83. https://doi.org/10.1586/14760584.2.2.269.
Acknowledgements
One of the authors Ms. Sunita Minz (SRF-RGNF) appreciates University Grants Commission, New Delhi, India for providing financial assistance. Authors are also grateful to Serum Institute of Pune, India for providing gift sample (rHBsAg). All India Institute of Medical Sciences (AIIMS, New Delhi, India) for providing electron microscopy facility and National Institute of Pharmaceutical Education and Research (NIPER, Mohali, India) for fluorescence spectroscopy and circular dichroism.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was carried out as per guidelines issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, Ministry of Social Empowerment and Justice, Government of India). The experimental protocol on animals was approved by the Institutional animal ethics committee (IAEC).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Minz, S., Pandey, R.S. Lipid A adjuvanted Chylomicron Mimicking Solid Fat Nanoemulsions for Immunization Against Hepatitis B. AAPS PharmSciTech 19, 1168–1181 (2018). https://doi.org/10.1208/s12249-017-0932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0932-9