Abstract
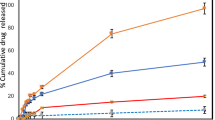
The aim of the present study was to evaluate the effectiveness of iontophoretic co-delivery of curcumin and anti-STAT3 siRNA using cationic liposomes against skin cancer. Curcumin was encapsulated in DOTAP-based cationic liposomes and then complexed with STAT3 siRNA. This nanocomplex was characterized for the average particle size, zeta-potential, and encapsulation efficiency. The cell viability studies in B16F10 mouse melanoma cells have shown that the co-delivery of curcumin and STAT3 siRNA significantly (p < 0.05) inhibited the cancer cell growth compared with either liposomal curcumin or STAT3 siRNA alone. The curcumin-loaded liposomes were able to penetrate up to a depth of 160 μm inside the skin after iontophoretic (0.47 mA/cm2) application. The in vivo efficacy studies were performed in the mouse model of melanoma skin cancer. Co-administration of the curcumin and STAT3 siRNA using liposomes significantly (p < 0.05) inhibited the tumor progression as measured by tumor volume and tumor weight compared with either liposomal curcumin or STAT3 siRNA alone. Furthermore, the iontophoretic administration of curcumin-loaded liposome-siRNA complex showed similar effectiveness in inhibiting tumor progression and STAT3 protein suppression compared with intratumoral administration. Taken together, cationic liposomes can be utilized for topical iontophoretic co-delivery of small molecule and siRNA for effective treatment of skin diseases.







Similar content being viewed by others
References
Ghosh TK, Abraham W, Jasti BR. Transdermal and topical drug delivery systems. Theory and practice of contemporary pharmaceutics: CRC Press; 2004. p. 423–55.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3(9):318–26.
Wang Y, Thakur R, Fan Q, Michniak B. Transdermal iontophoresis: combination strategies to improve transdermal iontophoretic drug delivery. Eur J Pharm Biopharm. 2005;60(2):179–91.
Benson HA. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv. 2005;2(1):23–33.
Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109–31.
Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res. 2009;8(2).
Finnin BC, Morgan TM. Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88(10):955–8.
Nanda A, Nanda S, Khan GN. Current developments using emerging transdermal technologies in physical enhancement methods. Curr Drug Deliv. 2006;3(3):233–42.
Mitragotri S. Devices for overcoming biological barriers: the use of physical forces to disrupt the barriers. Adv Drug Deliv Rev. 2013;65(1):100–3.
Ting WW, Vest CD, Sontheimer RD. Review of traditional and novel modalities that enhance the permeability of local therapeutics across the stratum corneum. Int J Dermatol. 2004;43(7):538–47.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68.
Gupta M, Agrawal U, Vyas SP. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv. 2012;9(7):783–804.
Neubert RH. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur J Pharm Biopharm. 2011;77(1):1–2.
Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322(1):60–6.
Souza JG, Dias K, Pereira TA, Bernardi DS, Lopez RF. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol. 2014;66(4):507–30.
Kajimoto K, Yamamoto M, Watanabe M, Kigasawa K, Kanamura K, Harashima H, et al. Noninvasive and persistent transfollicular drug delivery system using a combination of liposomes and iontophoresis. Int J Pharm. 2011;403(1):57–65.
Han I, Kim M, Kim J. Enhanced transfollicular delivery of adriamycin with a liposome and iontophoresis. Exp Dermatol. 2004;13(2):86–92.
Eljarrat-Binstock E, Orucov F, Aldouby Y, Frucht-Pery J, Domb AJ. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J Control Release. 2008;126(2):156–61.
Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(3):205–18.
Franceschi S, Levi F, Randimbison L, La Vecchia C. Site distribution of different types of skin cancer: new aetiological clues. Int J Cancer. 1996;67(1):24–8.
Balch CM, Soong S-J, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34.
La Porta CA. Drug resistance in melanoma: new perspectives. Curr Med Chem. 2007;14(4):387–91.
Teo PY, Cheng W, Hedrick JL, Yang YY. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev. 2016;98:41–63.
Jose A, Labala S, Venuganti VVK. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J Drug Target. 2016:1–15.
Labala S, Jose A, Chawla S, Khan MS, Bhatnagar S, Kulkarni OP, et al. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int J Pharm. 2017;525:407–17.
M-k K, G-j C, Lee H-s. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J Agric Food Chem. 2003;51(6):1578–81.
Ruby A, Kuttan G, Babu KD, Rajasekharan K, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94(1):79–83.
Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S, et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403(1):285–91.
Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17(1):71–80.
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2011;4(8):1158–71.
Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15.
Karewicz A, Bielska D, Gzyl-Malcher B, Kepczynski M, Lach R, Nowakowska M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf B Biointerfaces. 2011;88(1):231–9.
Geusens B, Lambert J, De Smedt S, Buyens K, Sanders N, Van Gele M. Ultradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytes. J Control Release. 2009;133(3):214–20.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809.
Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24(2):315–27.
Yang C-L, Liu Y-Y, Ma Y-G, Xue Y-X, Liu D-G, Ren Y, et al. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One. 2012;7(5):e37960.
Hervella P, Lozano V, Garcia-Fuentes M, Alonso MJ. Nanomedicine: new challenges and opportunities in cancer therapy. J Biomed Nanotechnol. 2008;4(3):276–92.
Guy RH, Kalia YN, Delgado-Charro MB, Merino V, Lopez A, Marro D. Iontophoresis: electrorepulsion and electroosmosis. J Control Release. 2000;64(1):129–32.
Venuganti VVK, Saraswathy M, Dwivedi C, Kaushik RS, Perumal OP. Topical gene silencing by iontophoretic delivery of an antisense oligonucleotide–dendrimer nanocomplex: the proof of concept in a skin cancer mouse model. Nano. 2015;7(9):3903–14.
Acknowledgements
This work was financially supported by a grant from Science and Engineering Research Board, Department of Science and Technology (DST) (SR/S0/HS-0059/2012), Government of India. Particle size analyzer and multimode plate reader were procured using a grant from Department of Science and Technology—fund for improvement of science and technology infrastructure (DST FIST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Electronic supplementary material
Esm 1
(DOCX 703 kb).
Rights and permissions
About this article
Cite this article
Jose, A., Labala, S., Ninave, K.M. et al. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech 19, 166–175 (2018). https://doi.org/10.1208/s12249-017-0833-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0833-y