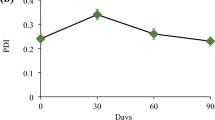
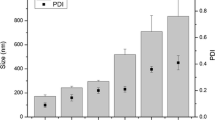
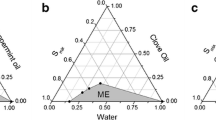
Abstract
Pomegranate peel and seeds have demonstrated to possess antioxidant compounds with potential application to protect the skin against the ultraviolet radiation damage. However, the photoprotection activity is dependent on the amount of these compounds that reach the viable skin layers. In this paper, we describe the in vitro skin permeation and retention of the major pomegranate peel polyphenols using Franz diffusion cells, after entrapping a ethyl acetate fraction (EAF) from Punica granatum peel extract into nanoemulsions (NEs) prepared with pomegranate seed oil (PSO) or medium chain triglyceride oil (MCT). The in vitro skin permeation of gallic acid (GA), ellagic acid (EA), and punicalagin (PC) was evaluated using a HPLC-DAD validated method. After 8 h of skin permeation, all polyphenol compounds were mostly retained in the skin and did not reach the receptor compartment. However, a 2.2-fold enhancement of the retained amount of gallic acid in the stratum corneum was verified after EAF-loaded NEs are applied, when compared with the free EAF. GA and EA were delivered to the viable epidermis and dermis only when nanoemulsions were applied onto the skin. The mean retained amounts of GA and EA in the EP and DE after applying the EAF-loaded PSO-NE were 1.78 and 1.36 μg cm−2 and 1.10 and 0.97 μg cm−2, respectively. Similar values were obtained after applying the EAF-loaded MCT-NE. The skin permeation results were supported by the confocal microscopy images. These results evidenced the promising application of nanoemulsions to deliver the pomegranate polyphenols into the deeper skin layers.


Similar content being viewed by others
References
Bito T, Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci. 2012;68(1):3–8. doi:10.1016/j.jdermsci.2012.06.006.
Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508(2):144–51. doi:10.1016/j.abb.2010.12.007.
Godic A, Poljak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxidative Med Cell Longev. 2014;2014:1–6. doi:10.1155/2014/860479.
Freitas JV, Praça FSG, Bentley MVLB, Gaspar LR. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int J Pharm. 2015;484(1–2):131–7. doi:10.1016/j.ijpharm.2015.02.062.
Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, et al. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm. 2000;199(1):39–47. doi:10.1016/S0378-5173(00)00358-6.
Manasathien J, Indrapichate K, Intarapichet K-O. Antioxidant activity and bioefficacy of pomegranate Punica granatum Linn. peel and seed extracts. Global J Pharmacol. 2012;6(2):131–41. doi:10.5829/idosi.gjp.2012.6.2.64226.
Fischer UA, Carle R, Kammerer DR. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011;127(2):807–21. doi:10.1016/j.foodchem.2010.12.156.
Fadavi A, Barzegar M, Azizi HM. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J Food Compos Anal. 2006;19:676–80. doi:10.1016/j.jfca.2004.09.002.
El-Nemr SE, Ismail IA, Ragab M. Chemical composition of juice and seeds of pomegranate fruit. Nahrung. 2006;34(7):601–6. doi:10.1002/food.19900340706.
Qu W, Pan Z, Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng. 2010;99(1):16–23. doi:10.1016/j.jfoodeng.2010.01.020.
Manasathien J, Kupittayanant S, Indrapichate K. Protective efficacy of pomegranate (Punica granatum Linn., Punicaceae) peel ethanolic extracts on UVB-irradiated rat skin. Am Eurasian J Toxicol Sci. 2011;3(4):250–8.
Kasai K, Yoshimura M, Koga T, Arii M, Kawasaki S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J Nutr Sci Vitaminol. 2006;52(5):383–8.
Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J Agric Food Chem. 2008;56(18):8434–41. doi:10.1021/jf8005307.
Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interf Sci. 2004;108–109:145–9. doi:10.1016/j.cis.2003.10.026.
Kong M, Chen XG, Kweon DK, Park HJ. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr Polym. 2011;86(2):837–43. doi:10.1016/j.carbpol.2011.05.027.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi:10.1016/j.jconrel.2017.03.008.
Baccarin T, Lemos-Senna E. Pomegranate seed oil nanoemulsions encapsulating pomegranate peel polyphenol-rich ethyl acetate fraction: development and antioxidant assessment. J Nanopharm Drug Deliv. 2014;2(4):333–43. doi:10.1166/jnd.2014.1068.
Baccarin T, Mitjans M, Lemos-Senna E, Vinardell MP. Protection against oxidative damage in human erythrocytes and preliminary photosafety assessment of Punica granatum seed oil nanoemulsions entrapping polyphenol-rich ethyl acetate fraction. Toxicol in Vitro. 2015;30(1 part B):421–8. doi:10.1016/j.tiv.2015.09.020.
Baccarin T, Mitjans M, Ramos D, Lemos-Senna E, Vinardell MP. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J Photochem Photobiol B. 2015;153:127–36. doi:10.1016/j.jphotobiol.2015.09.005.
Jacobs GA, Gerber M, Malan MM, du Preez JL, Fox LT, du Plessis J. Topical delivery of acyclovir and ketoconazole. Drug Deliv. 2016;23(2):631–41. doi:10.3109/10717544.2014.933283.
Escobar-Chávez JJ, Merino-Sanjuán V, Lópes-Cervantes M, Uuban-Morlan Z, Piñón-Segundo E, Quintanar-Guerrero D, et al. The tape stripping technique as a method for drug quantification in skin. J Pharm Pharm Sci. 2008;11:104–30. doi:10.18433/J3201Z.
ICH. International Conference on Harmonization of Technical Requeriments for Registration of Pharmaceuticals for Human Use, validation of analytical procedures: text and methodology. Geneva, Switzerland 2005.
Brasil. Resolution RE 899 “Guide for analytical and bio-analitycal method validation”. Anvisa - Agência Nacional de Vigilância Satinária 2003.
Jarzycka A, Lewinska A, Gancarz R, Wilk KA. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J Photochem Photobiol B. 2013;128:50–7. doi:10.1016/j.jphotobiol.2013.07.029.
Nichols J, Katiyar S. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol res. 2010;302(2):71–83. doi:10.1007/s00403-009-1001-3.
Ratz-Łyko A, Arct J, Majewski S, Pytkowska K. Influence of polyphenols on the physiological processes in the skin. Phytother res. 2015;29(4):509–17. doi:10.1002/ptr.5289.
Shakeel F, Shafiq S, Haq N, Alanazi FK, Alsarra IA. Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: an overview. Expert Opin Drug Deliv. 2012;9(8):953–74. doi:10.1517/17425247.2012.696605.
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–10. doi:10.1016/j.cocis.2005.06.004.
Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interf Sci. 2004;108–109:303–18. doi:10.1016/j.cis.2003.10.023.
Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification:solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241–51.
Montenegro L, Carbone C, Puglisi G. Vehicle effects on in vitro release and skin permeation of octylmethoxycinnamate from microemulsions. Int J Pharm. 2011;405(1–2):162–8. doi:10.1016/j.ijpharm.2010.11.036.
Barradas TN, Senna JP, Cardoso SA, Nicoli S, Padula C, Santi P, et al. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: permeation and stability studies. Eur J Pharm Biopharm. 2017;116:38-50. doi:10.1016/j.ejpb.2016.11.018.
Parra A, Clares B, Rosselló A, Garduño-Ramírez ML, Abrego G, García ML, et al. Ex vivo permeation of carprofen from nanoparticles: a comprehensive study through human, porcine and bovine skin as anti-inflammatory agent. Int J Pharm. 2016;501(1–2):10–7. doi:10.1016/j.ijpharm.2016.01.056.
Dick IP, Scott RC. Pig ear skin as an in vitro model for human skin permeability. J Pharm Pharmacol. 1992;44(8):640–5. doi:10.1111/j.2042-7158.1992.tb05485.x.
Sekkat N, Kalia YN, Guy RH. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J Pharm Sci. 2002;91(11):2376–81. doi:10.1002/jps.10220.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv rev. 2012;64:128–37. doi:10.1016/j.addr.2012.09.032.
Friedrich RB, Kann B, Coradini K, Offerhaus HL, Beck RCR, Windbergs M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur J Pharm Sci. 2015;78:204–13. doi:10.1016/j.ejps.2015.07.018.
Mo J, Kaewnopparat N, Songkro S, Panichayupakaranant P, Reanmongkol W. Physicochemical properties, in vitro release and skin permeation studies of a topical formulation of standardized pomegranate rind extract. Pak J Pharm Sci. 2015;28(1):29–36.
Junyaprasert VB, Singhsa P, Jintapattanakit A. Influence of chemical penetration enhancers on skin permeability of ellagic acid-loaded niosomes. Asian J Pharm Sci. 2013;8(2):110–7. doi:10.1016/j.ajps.2013.07.014.
Chatelain E, Gabard B, Surber C. Skin penetration and sun protection factor of five UV filters: effect of the vehicle. Skin Pharmacol Physiol. 2003;16(1):28–35. doi:10.1159/000068291.
Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9. doi:10.1034/j.1600-0625.2000.009003165.x.
Alonso C, Rubio L, Touriño S, Martí M, Barba C, Fernández-Campos F, et al. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic Biol med. 2014;75:149–55. doi:10.1016/j.freeradbiomed.2014.07.014.
Acknowledgements
The authors acknowledge the financial support from Coordination for the Improvement of Higher Education Personnel (CAPES-DS). The authors would like to thank Msc. André O’Reilly Beringhs for his technical assistance in microtome-cryostat. The authors also would like to thank the Laboratório Central de Microscopia Eletrônica-LCME (Universidade Federal de Santa Catarina, Florianópolis, Brazil) for the confocal images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
According to the Animal Ethics Committee of Federal University of Santa Catarina, since we were dealing with porcine cadavers in the permeation/retention experiments, there was no need to protocol submission.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baccarin, T., Lemos-Senna, E. Potential Application of Nanoemulsions for Skin Delivery of Pomegranate Peel Polyphenols. AAPS PharmSciTech 18, 3307–3314 (2017). https://doi.org/10.1208/s12249-017-0818-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0818-x