Abstract
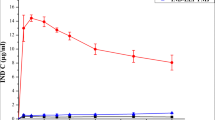
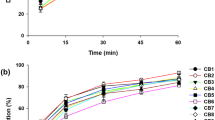
The aim of this study was to investigate the influence of factors such as carrier type, drug/carrier ratio, binary carriers, and preparation method on the dissolution of an insoluble drug, indomethacin (IM), under supersaturation conditions. Using a solvent evaporation (SE) method, poloxamer 188 and PVP K30 showed better dissolution among the selected carriers. Furthermore, as the ratio of carriers increased (drug/carrier ratio from 1:0.5 to 1:2), the dissolution rate increased especially in almost two times poloxamer 188 solid dispersions (SDs), while the reverse results were observed for PVP K30 SDs. For the binary carrier SD, a lower dissolution was found. Under hot melt extrusion (HME), the dissolution of poloxamer 188 SD and PVP K30 SD was 0.83- and 0.94-folds lower than that using SE, respectively, while the binary carrier SD showed the best dissolution. For poloxamer 188 SDs, the drug’s crystal form changed when using SE, while no crystal form change was observed using HME. IM was amorphous in PVP K30 SDs prepared by both methods. For binary carrier systems, amorphous and crystalline drugs coexisted in SD using SE, and negligible amorphous IM was in SD using HME. This study indicated that a higher amorphous proportion in SD did not correlate with higher dissolution rate, and other factors, such as carrier type, particle size, and density, were also critical.





















Similar content being viewed by others
References
Vasconcelos T, Samento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2008;39(25):1068–75.
Timpe C, Forschung L. Strategies for formulation development of poorly water-soluble drug candidates—a recent perspective. Am Pharm Rev. 2007;10:104–9.
Löbenberg R, Amidon G. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Sarode AL, Wang P, Obara S, Worthen DR. Supersaturation, nucleation, and crystal growth during single- and biphasic dissolution of amorphous solid dispersions: polymer effects and implications for oral bioavailability enhancement of poorly water soluble drugs. Eur J Pharm Biopharm. 2014;86(3):351–60.
Bikiaris D. Solid dispersions, part II: new strategies in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2011;8(12):1663–80.
Alam MA, Ali R, FI AI-J, AL-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9(11):1419–40.
Al-Obaidi H, Ke P, Brocchini S, Buckton B. Characterization and stability of ternary solid dispersions with PVP and PHPMA. Int J Pharm. 2011;419(1–2):20–7.
Martins RM, Siqueira S, Tacon LA, Freitas LAP. Microstructured ternary solid dispersions to improve carbamazepine solubility. Powder Technol. 2012;215(1):156–65.
Mahmah O, Tabbakh R, Kelly A, Paradkar A. A comparative study of the effect of spray drying and hot-melt extrusion on the properties of amorphous solid dispersions containing felodipine. J Pharm Pharmacol. 2014;66(2):275–84.
Srinarong P, Waard HD, Frijlink HW, Hinrichs WLJ. Improved dissolution behavior of lipophilic drugs by solid dispersions: the production process as starting point for formulation considerations. Expert Opin Drug Deliv. 2011;8(9):1121–40.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10.
Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM, Rades T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int J Pharm. 2007;336(1):22–34.
Dalsin MC, Tale S, Reineke TM. Solution-state polymer assemblies influence BCS class II drug dissolution and supersaturation maintenance. Biomacromolecules. 2014;15(2):500–11.
Newa M, Bhandari KH, Li DX, Kwon TH, Kim JA, Yoo BK, et al. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int J Pharm. 2007;343(1–2):228–37.
Maulvi FA, Dalwadi SJ, Thakkar VT, Soni TG, Gohel MC, Gandhi TR. Improvement of dissolution rate of aceclofenac by solid dispersion technique. Powder Technol. 2011;207(1–3):47–54.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Petralito S, Zanardi I, Memoli A, Cristina M, Millucci V, Travagli V. Apparent solubility and dissolution profile at non-sink conditions as quality improvement tools. Promising Pharmaceuticals. 2012;5:83–100.
Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267(1–2):79–91.
Overhoff KA, Moreno A, Miller DA, Johnston KP, Williams RO III. Solid dispersions of itraconazole and enteric polymers made by ultra-rapid freezing. Int J Pharm. 2007;336(1):122–32.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9.
Valizadeh H, Nokhodchi A, Qarakhani N, Zakeri-Milani P, Azarmi S, Hassanzadeh D, et al. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin, and Eudragit E100. Drug Dev Ind Pharm. 2004;30(3):303–17.
Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical; 2009. p. 506–9.
Vippagunta SR, Maul KA, Tallavajhala S, Grant DJW. Solid state characterization of nifedipine solid dispersion. Int J Pharm. 2002;236(1–2):111–23.
Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion processs as a means to manufacture solid dispersion/solution. J Pharm Sci. 2005;94(11):2463–74.
Yoshioka M, Hancock BC, Zografi G. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipiatates. J Pharm Sci. 1995;84(8):983–6.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56.
Frizon F, Eloy JDO, Donaduzzi CM, Mitsui ML, Marchetti JM. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013;235:532–9.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14(12):1691–8.
Matsumoto T, Zografi G. Physical properties of solid molecular dispersions of indomethacin with poly(vinylpyrrolidone) and poly (vinylpyrrolidone-co-vinyl-acetate) in relation to indomethacin crystallization. Pharm Res. 1999;16(11):1722–8.
Otsuka M, Kato F, Matsuda Y. Determination of indomethacin polymorphic contents by chemometric near-infrared spectroscopy and conventional powder X-ray diffractometry. Analyst. 2001;126(9):1578–82.
Pan X, Julian T, Augsburger L. Quantitative measurement of indomethacin crystallinity in indomethacin-silica gel binary system using differential scanning calorimetry and X-ray powder diffractometry. AAPS Pharm Sci Tech. 2006;7(1):E72–8.
Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J. Causative factors behind poloxamer 188 (Pluronic F68, FlocorTM)-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. BBA-GEN Subjiects. 2004;1689(2):103–13.
Chutimaworapan S, Ritthidej GC, Yonemochi E, Oguchi T, Yamamoto K. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm. 2000;26(11):1141–50.
Karavas E, Georgarakis E, Sigalas MP, Avgoustakis K, Bikiaris D. Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug-polymer interactions. Eur J Pharm Biopharm. 2007;66(3):334–47.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Sci. 2000;50(1):47–60.
Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131–44.
Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271(1–2):281–6.
Wang X, Michoel A, Mooter GV. Solid state characteristics of ternary solid dispersions composed of PVP VA64, Myrj 52 and itraconazole. Int J Pharm. 2005;303:54–61.
Janssens S, Nagels S, Armas HN, Autry WD, Schepdael AV, Mooter GV. Formulation and characterization of ternary solid dispersions made up of Itraconazole and two excipients, TPGS 1000 and PVPVA 64, that were selected based on a supersaturation screening study. Eur J Pharm Biopharm. 2008;69:158–66.
Follonier N, Doelker E, Cole ET. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained release pellets prepared using polymeric materials. J Control Release. 1995;36(3):243–50.
Andrews GP, Abu-Diak O, Kusmanto F, Homsby P, Hui Z, Jones DS. Physicochemical characterization and drug-release properties of celecoxib hot-melt extruded glass solutions. J Pharm Pharmacol. 2010;62(11):1580–90.
Hancock BC, Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000;17(4):397–404.
Dong Z, Chatterji A, Sandhu H, Choi DS, Chokshi H, Shah N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int J Pharm. 2008;355(1–2):141–9.
Acknowledgements
The authors would like to thank Professor Li Tong and Professor Wang Lihong for their helpful discussions. The Science and Technology Research and Development Project of Shiyan City in the year 2016 (No.: 16Y20) funded this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
The authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Zhang, W., Zhang, Cn., He, Y. et al. Factors Affecting the Dissolution of Indomethacin Solid Dispersions. AAPS PharmSciTech 18, 3258–3273 (2017). https://doi.org/10.1208/s12249-017-0813-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0813-2