Abstract
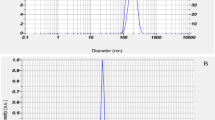
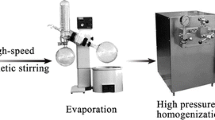
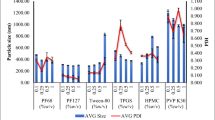
The clinical potential of naringenin (NRG) is compromised due to its poor aqueous solubility and low oral bioavailability. The study is aimed at addressing these issues by means of naringenin nanosuspensions (NRG-NS) formulated using polyvinylpyrrolidone (PVP K-90) as stabiliser via antisolvent sonoprecipitation method. Optimisation of sonication time, drug concentration and stabilisers was done based on particle size. Characterisation of pure NRG and NRG-NS was carried out by scanning electron microscopy, differential scanning calorimetry (DSC), x-ray powder diffractometry (XRD) and Fourier transform infrared spectroscopy (FTIR). In vitro dissolution, intestinal absorption by non-everted rat intestinal sac model and in situ single pass intestinal perfusion techniques were performed for further investigation. Nanosuspensions prepared using PVP K-90 lead to minimum particle size (117 ± 5 nm) with zeta potential of −14.6 ± 5.6 mV. The particle size was affected by increasing sonication time, concentration of stabiliser and drug. Nanosizing process converted the crystalline drug into amorphous form as predicted from DSC and XRD patterns. FTIR demonstrated the formation of hydrogen bonds between drug and polymer. NRG-NS displayed a higher dissolution amount (91 ± 4.4% during 60 min) compared to NRG powder (42 ± 0.41%). The apparent and effective permeability of NRG-NS was increased as compared to the pure NRG. The in vivo pharmacokinetics demonstrated that the C max and AUC0–24 h values of NRG-NS were approximately 2- and 1.8-fold superior than the pure drug. Hence, overall results confirmed nanosuspensions as promising approach for NRG delivery with high absorption in gastrointestinal tract, improved dissolution and oral bioavailability.










Similar content being viewed by others
References
Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2014;1–13.
Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Forum Nutr. 2013;5(9):3367–87.
Kanaze F, Bounartzi M, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61(4):472–7.
Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–41.
Wang MJ, Chao PDL, Hou YC, Hsiu SL. Pharmacokinetics and conjugation metabolism of naringin and naringenin in rats after single dose and multiple dose administrations. J Food Drug Anal. 2006;14(3):247–53.
Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm Res. 2009;26(4):893–902.
Shulman M, Cohen M, Soto-Gutierrez A, Yagi H, Wang H, Goldwasser J, Lee-Parsons CW, Benny-Ratsaby O, Yarmush ML, et al. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-Î2-cyclodextrin. PLoS One. 2011;6(4) doi:10.1371/journal.pone.0018033.
Xu XR, Yu HT, Hang L, Shao Y, Ding SH, Yang XW. Preparation of naringenin/β-cyclodextrin complex and its more potent alleviative effect on choroidal neovascularization in rats. Biomed Res Int. 2014;1–9.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61.
Semalty A, Semalty M, Singh D, Rawat MSM. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J Incl Phenom Macrocycl Chem. 2010;67(3–4):253–60.
Ji P, Yu T, Liu Y, Jiang J, Xu J, Zhao Y, Hao Y, Qiu Y, Zhao W, et al. Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des Dev Ther. 2016;10:911–25.
Tsai MJ, Huang YB, Fang JW, Fu YS, Wu PC. Preparation and characterization of naringenin-loaded elastic liposomes for topical application. PLoS One. 2015;10(7) doi:10.1371/journal.pone.0131026.
Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: a comparative study. J Appl Polym Sci. 2006;102(1):460–71.
Suseela P, Premkumar K, Saraswathy SD. Formulation, characterization and pharmacokinetic evaluation of naringenin-loaded gastroretentive mucoadhesive polymeric nanosystem for oral drug delivery. J drug deliv ther. 2015;5(2):107–14.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–96.
Karadag A, Ozcelik B, Huang Q. Quercetin nanosuspensions produced by high-pressure homogenization. J Agric Food Chem. 2014;62(8):1852–9.
Mauludin R, Muller RH. Preparation and storage stability of rutin nanosuspensions. J Pharm Investig. 2013;43(5):395–404.
Hong C, Dang Y, Lin G, Yao Y, Li G, Ji G, Shen H, Xie Y. Effects of stabilizing agents on the development of myricetin nanosuspension and its characterization: an in vitro and in vivo evaluation. Int J Pharm. 2014;477(1):251–60.
Mishra PR, Al Shaal L, Muller RH, Keck CM. Production and characterization of hesperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371(1):182–9.
Keck CM, Muller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, Wang Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495(2):738–49.
Lonare AA, Patel SR. Antisolvent crystallization of poorly water soluble drugs. Int J Chem Eng Appl. 2013;4(5):337–41.
He S, Yang H, Zhang R, Li Y, Duan L. Preparation and in vitro, in vivo evaluation of teniposide nanosuspensions. Int J Pharm. 2015;478(1):131–7.
Dong Y, Ng WK, Hu J, Shen S, Tan RB. A continuous and highly effective static mixing process for antisolvent precipitation of nanoparticles of poorly water-soluble drugs. Int J Pharm. 2010;386(1):256–61.
Leroueil Le Verger M, Fluckiger L, Kim YI, Hoffman M, Maincent P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46(2):137–43.
Liu D, Xu H, Tian B, Yuan K, Pan H, Ma S, Yang X, Pan W. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech. 2012;13(1):295–304.
Neerati P, Bedada SK. Effect of diosmin on the intestinal absorption and pharmacokinetics of fexofenadine in rats. Pharmacol Rep. 2015;67(2):339–44.
Sahu BP, Das MK. Nanosuspension for enhancement of oral bioavailability of felodipine. Appl Nanosci. 2014;4(2):189–97.
Jiang T, Han N, Zhao B, Xie Y, Wang S. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug Dev Ind Pharm. 2012;38(10):1230–9.
Li H, Wang J, Bao Y, Guo Z, Zhang M. Rapid sonocrystallization in the salting-out process. J Cryst Growth. 2003;247(1):192–8.
Belkacem N, Salem MAS, Alkhatib HS. Effect of ultrasound on the physico-chemical properties of poorly soluble drugs: antisolvent sonocrystallization of ketoprofen. Powder Technol. 2015;285:16–24.
Ozaki S, Kushida I, Yamashita T, Hasebe T, Shirai O, Kano K. Inhibition of crystal nucleation and growth by water-soluble polymers and its impact on the supersaturation profiles of amorphous drugs. J Pharm Sci. 2013;102(7):2273–81.
Bi Y, Liu J, Wang J, Hao J, Li F, Wang T, Sun HW, Guo F. Particle size control and the interactions between drug and stabilizers in an amorphous nanosuspension system. J Drug Deliv Sci Technol. 2015;29:167–72.
Sepassi S, Goodwin D, Drake A, Holland S, Leonard G, Martini L, Lawrence M. Effect of polymer molecular weight on the production of drug nanoparticles. J Pharm Sci. 2007;96(10):2655–66.
Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364(1):64–75.
Dong Y, Ng WK, Shen S, Kim S, Tan RB. Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation. Int J Pharm. 2009;375(1):84–8.
Xia D, Quan P, Piao H, Piao H, Sun S, Yin Y, Cui F. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40(4):325–34.
Chen Y, Liu J, Yang X, Zhao X, Xu H. Oleanolic acid nanosuspensions: preparation, in-vitro characterization and enhanced hepatoprotective effect. J Pharm Pharmacol. 2005;57(2):259–64.
Kakran M, Sahoo NG, Li L, Judeh Z. Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. 2012;223:59–64.
Zeng L, Weber AP. Aerosol synthesis of nanoporous silica particles with controlled pore size distribution. J Aerosol Sci. 2014;76:1–12.
Patravale V, Kulkarni R. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–40.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Dhumal RS, Biradar SV, Yamamura S, Paradkar AR, York P. Preparation of amorphous cefuroxime axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. Eur J Pharm Biopharm. 2008;70(1):109–15.
Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406(1):145–52.
Ruan LP, Chen S, Yu BY, Zhu DN, Cordell G, Qiu S. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur J Med Chem. 2006;41(5):605–10.
Attari Z, Bhandari A, Jagadish P, Lewis S. Enhanced ex vivo intestinal absorption of olmesartan medoxomil nanosuspension: preparation by combinative technology. Saudi Pharm J. 2016;24(1):57–63.
He J, Han Y, Xu G, Yin L, Neubi MN, Zhou J, Ding Y. Preparation and evaluation of celecoxib nanosuspensions for bioavailability enhancement. RSC Adv. 2017;7(22):13053–64.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51(1):9–17.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 425 kb).
Rights and permissions
About this article
Cite this article
Gera, S., Talluri, S., Rangaraj, N. et al. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 18, 3151–3162 (2017). https://doi.org/10.1208/s12249-017-0790-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0790-5