Abstract
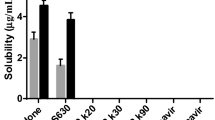
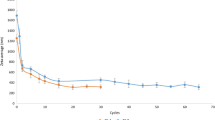
Efavirenz is a fundamental drug in the HIV therapy; however, it has a low bioavailability due to low water solubility. Particle nanonization should enhance its dissolution and therefore its bioavailability. Nanocrystallization is a promising technique for preparing drug nanocrystals. A solution containing efavirenz (EFV) and methanol was added to an aqueous solution of particle stabilizers, under sonication. The adequate polymer stabilizer and its concentration and drug load were evaluated. Particle size and zeta potential of suspensions were measured. Nanosuspensions were freeze-dried and the resulting powder was characterized by some techniques, with special attention to dissolution. Particle size and zeta potential analysis showed that HMPC and PVP were the most suitable polymers. All samples prepared with these stabilizers had nanosized particles and proper zeta potential; however, sedimentation and particle growth were detected with Turbiscan™. Time-related destabilization occurred when the lowest polymer concentration of 20% was used. SEM analysis of the dried powder shows film formation for suspensions with 40% of polymer and particle aggregation in samples with less polymer. Dissolution profiles of samples were higher than EFV raw material, although the lower the polymer concentration, the higher the dissolution.







Similar content being viewed by others
Abbreviations
- AIDS:
-
Acquired immunodeficiency syndrome
- ATR:
-
Attenuated total reflectance
- BCS:
-
Biopharmaceutics Classification System
- BS:
-
Backscattering
- EFV:
-
Efavirenz
- HIV:
-
Human immunodeficiency virus
- HPC:
-
Hydroxypropyl cellulose
- HPMC:
-
Hydroxypropyl methylcellulose
- IDR:
-
Intrinsic dissolution rate
- IR:
-
Infrared
- PDI:
-
Polydispersity index
- PVP:
-
Polyvinylpyrrolidone
- PXRD:
-
Powder X-ray diffraction
- SEM:
-
Scanning electron microscopy
- SLS:
-
Sodium lauryl sulfate
- TSI:
-
Turbiscan stability index
- ΔBS:
-
Backscattering variation
- ζ :
-
Zeta potential
References
Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, Richter C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61:148–54.
Chiappetta DA, Facorro G, de Celis ER, Sosnik A. Synergistic encapsulation of the anti-HIV agent efavirenz within mixed poloxamine/poloxamer polymeric micelles. Nanomedicine. 2011;7:624–37.
Patel GV, Patel VB, Pathak A, Rajput SJ. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40:80–91.
Chiappetta DA, Hocht C, Taira C, Sosnik A. Oral pharmacokinetics of the anti-HIV efavirenz encapsulated within polymeric micelles. Biomaterials. 2011;32:2379–87.
Cristofoletti R, Nair A, Abrahamsson B, Groot DW, Kopp S, Langguth P, et al. Biowaiver monographs for immediate release solid oral dosage forms: efavirenz. J Pharm Biomed Anal. 2013;102:318–29.
Cho E, Cho W, Cha K-H, Park J, Kim M-S, Kim J-S, et al. Enhanced dissolution of megestrol acetate microcrystals prepared by antisolvent precipitation process using hydrophilic additives. Int J Pharm. 2010;396:91–8.
Fandaruff C, Rauber GS, Araya-sibaja AM, Pereira RN, Campos CEMD, Rocha HVA, et al. Polymorphism of anti-HIV drug efavirenz: investigations on thermodynamic and dissolution properties. Cryst Growth Des. 2014;14:4968–75.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:151–70.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30:307–24.
Patel AP, Patel JK, Patel KS, Deshmukh AB, Mishra BR. A review on drug nanocrystal a carrier free drug delivery. Int J Res Ayurveda Pharm. 2011;2:448–58.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160:418–30.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380:216–22.
de Waard H, Grasmeijer N, Hinrichs WLJ, Eissens AC, Pfaffenbach PPF, Frijlink HW. Preparation of drug nanocrystals by controlled crystallization: application of a 3-way nozzle to prevent premature crystallization for large scale production. Eur J Pharm Sci. 2009;38:224–9.
Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453:126–41.
Jain S, Sharma JM, Agrawal AK, Mahajan RR. Surface stabilized efavirenz nanoparticles for oral bioavailability enhancement. J Biomed Nanotechnol. 2013;9:1862–74.
Ye X, Patil H, Feng X, Tiwari RV, Lu J, Gryczke A, et al. Conjugation of hot-melt extrusion with high-pressure homogenization: a novel method of continuously preparing nanocrystal solid dispersions. AAPS PharmSciTech. 2015; doi:10.1208/s12249-015-0389-7.
da Costa MA, Seiceira RC, Rodrigues CR, Hoffmeister CRD, Cabral LM, Rocha HVA. Efavirenz dissolution enhancement I: co-micronization. Pharmaceutics. 2013;5:1–22.
Costa MA, Lione VOF, Cabral LM, Rocha HVA. Efavirenz dissolution enhancement II: aqueous co-spray-drying. Int J Pharm Sci Res. 2015;6:3807–20.
Hoffmeister CRD, Fandaruff C, Costa MA, Cabral LM, Pitta LR, Bilatto SER et al. Efavirenz dissolution enhancement III: colloid milling, pharmacokinetics and electronic tongue evaluation. Submited Artic.
Sze A, Erickson D, Ren L, Li D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J Colloid Interface Sci. 2003;261:402–10.
Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406:145–52.
Choi J-Y, Yoo JY, Kwak H-S, Uk Nam B, Lee J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr Appl Phys. 2005;5:472–4.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172:1126–41.
Carrique F, Arroyo F, Delgado A. Effect of a dynamic stern layer on the sedimentation velocity and potential in a dilute suspension of colloidal particles. J Colloid Interface Sci. 2000;227:212–22.
Nasser MS, James AE. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions. Sep Purif Technol. 2006;52:241–52.
Sawant SV, Kadam VJ, Jadhav KR, Sankpal SV. Drug nanocrystals: novel technique for delivery of poorly soluble drugs. Int J Sci Innov Discov. 2011;1:1–15.
Liu G, Zhang D, Jiao Y, Zheng D, Liu Y, Duan C, et al. Comparison of different methods for preparation of a stable riccardin D formulation via nano-technology. Int J Pharm. 2012;422:516–22.
Mengual O. TURBISCAN MA 2000: multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta. 1999;50:445–56.
Kang W, Xu B, Wang Y, Li Y, Shan X, An F, et al. Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloids Surfaces A Physicochem Eng Asp. 2011;384:555–60.
Gomes EC d L, Mussel WN, Resende JM, Fialho SL, Barbosa J, Yoshida MI. Chemical interactions study of antiretroviral drugs efavirenz and lamivudine concerning the development of stable fixed-dose combination formulations for AIDS treatment. J Brazilian Chem Soc. 2013;24:573–9.
Mahapatra AK, Murthy PN. Solubility and dissolution rate enhancement of efavirenz by inclusion complexation and liquid anti-solvent precipitation technique. J Chem Pharm Res. 2014;6:1099–106.
Stuart BH. Infrared spectroscopy: fundamentals and applications. 2004. doi:10.1002/0470011149.
Shankar S, Rhim JW. Preparation of nanocellulose from micro-crystalline cellulose: the effect on the performance and properties of agar-based composite films. Carbohydr Polym. 2016;135:18–26.
Laot CM, Marand E, Oyama HT. Spectroscopic characterization of molecular interdiffusion at a poly(vinyl pyrrolidone)/vinyl ester interface. Polymer (Guildf). 1999; 40. doi:10.1016/S0032-3861(98)80003-7.
Mahapatra S, Thakur TS, Joseph S, Varughese S, Desiraju GR. New solid state forms of the anti-HIV drug efavirenz. Conformational flexibility and high Z’ issues. Cryst Growth Des. 2010;10:3191–202.
Blachére JR, Brittain HG. X-ray diffraction methods for the characterization of solid pharmaceutical materials. In: Adeyeye M, Brittain HG, editors. Drugs and pharmaceutical sciences: preformulation in solid dosage form development. New York: Informa Healthcare; 2008. p. 229–52.
Matteucci ME, Hotze MA, Johnston KP, Williams RO. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006;22:8951–9.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Understanding polymer properties important for crystal growth inhibition-impact of chemically diverse polymers on solution crystal growth of ritonavir. Cryst Growth Des. 2012;12:3133–43.
Acknowledgments
The authors want to acknowledge the Laboratory of Pharmaceutical Technology (Farmanguinhos, Fiocruz), the Laboratory of X-Ray Diffraction (UFF), the Technological Characterization Sector from the Mineral Technology Center (CETEM), the Laboratory of the Chemical Engineering Department (UFRJ), the Laboratory of Polymerization Engineering (UFRJ), the Department of Natural Products (Farmanguinhos, Fiocruz), and the Platform of Analytical Methods (Farmanguinhos, Fiocruz).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(GIF 103 kb)
Rights and permissions
About this article
Cite this article
Sartori, G.J., Prado, L.D. & Rocha, H.V.A. Efavirenz Dissolution Enhancement IV—Antisolvent Nanocrystallization by Sonication, Physical Stability, and Dissolution. AAPS PharmSciTech 18, 3011–3020 (2017). https://doi.org/10.1208/s12249-017-0781-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0781-6