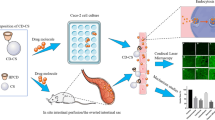
Abstract
Most therapeutic proteins are classified as class III drugs according to the Biopharmaceutical Classification System means that the low permeability across the intestinal epithelium is the rate-limited step for their oral absorption. Cationic chitosan nanoparticles have been found to open the tight junctions between epithelial cells. On the other hand, bioadhesive delivery devices could prolong the gastrointestinal residence time. In the present study, we developed a novel nano-reservoir bioadhesive tablets that combining the advantages of cationic nanoparticles and bioadhesive delivery devices anticipated achieving effective transport of sufficient protein drugs across the intestinal epithelium. The nano-reservoir in bioadhesive tablets was composed of chitosan nanoparticles (CS-NPs) loading a model protein drug bovine serum albumin (BSA). The formula of bioadhesive tablets was optimized by using rotatable central composite design and response surface methodology. The nano-reservoir, BSA-loaded CS-NPs, had an average particle diameter of 312.5 ± 12.89 nm and zeta-potential value of 26.76 ± 3.56 mV. Carboxymethyl chitosan added to the formula significantly ameliorated the tight junction damage of the Caco-2 cell monolayer induced by CS-NPs, meanwhile maintained the high transport efficiency of BSA. Permeability study exhibited that these nano-reservoir bioadhesive tablets combining the advantages of cationic nanoparticles and bioadhesive tablets significantly enhanced BSA transport through rabbit small intestine in comparison with either conventional bioadhesive tablets or CS-NPs. Therefore, these nano-reservoir bioadhesive tablets provided a great potential dosage form design for the oral delivery of protein drugs.







Similar content being viewed by others
References
Niu Z, Conejos-Sánchez I, Griffin BT, O’Driscoll CM, Alonso MJ. Lipid-based nanocarriers for oral peptide delivery. Adv Drug Deliv Rev. 2016. doi:10.1016/S0169-409X(16)30100-4.
Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi:10.1038/nrd2399.
Birch JR, Onakunle Y. Biopharmaceutical proteins: opportunities and challenges. Methods Mol Biol. 2005;308:1–16. doi:10.1385/159259-922-2.
Werle M, Makhlof A, Takeuchi H. Oral protein delivery: a patent review of academic and industrial approaches. Recent Pat Drug Deliv Formul. 2009;3:94–104. doi:10.2174/187221109788452221.
Herrero EP, Alonso MJ, Csaba N. Polymer-based oral peptide nanomedicines. Ther Deliv. 2012;3:657–68. doi:10.4155/tde.12.40.
Yun Y, Cho YW, Park K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Deliv Rev. 2013;65:822–32. doi:10.1016/j.addr.2012.10.007.
Sonia TA, Sharma CP. Chitosan and its derivatives for drug delivery perspective. Adv Polym Sci. 2011;243:23–54. doi:10.1007/12_2011_117.
Chen MC, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung MF, et al. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev. 2013;65:965–79. doi:10.1016/j.addr.2012.10.010.
Schulz JD, Gauthier MA, Leroux JC. Improving oral drug bioavailability with polycations? Eur J Pharm Biopharm. 2015;97:427–37. doi:10.1016/j.ejpb.2015.04.025.
Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target. 2012;20:291–300. doi:10.3109/1061186X.2011.654121.
Garcia-Fuentes M, Alonso MJ. Chitosan-based drug nanocarriers: where do we stand? J Control Release. 2012;161:496–504. doi:10.1016/j.jconrel.2012.03.017.
Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull (Tokyo). 2010;58:1423–30. doi:10.1248/cpb.58.1423.
Zhang J, Zhu X, Jin Y, Shan W, Huang Y. Mechanism study of cellular uptake and tight junction opening mediated by goblet cell-specific trimethyl chitosan nanoparticles. Mol Pharm. 2014;11:1520–32. doi:10.1021/mp400685v.
Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. doi:10.2353/ajpath.2010.100371.
Fischer KE, Jayagopal A, Nagaraj G, Daniels RH, Li EM, Silvestrini MT, et al. Nanoengineered surfaces enhance drug loading and adhesion. Nano Lett. 2011;11:1076–81. doi:10.1021/nl103951e.
Ameye D, Voorspoels J, Foreman P, Tsai J, Richardson P, Geresh S, et al. Ex vivo bioadhesion and in vivo testosterone bioavailability study of different bioadhesive formulations based on starch-g-poly(acrylic acid) copolymers and starch/poly(acrylic acid) mixtures. J Control Release. 2002;79:173–82. doi:10.1016/S0168-3659(01)00539-9.
Sharma S, Mukkur TK, Benson HA, Chen Y. Enhanced immune response against pertussis toxoid by IgA-loaded chitosan–dextran sulfate nanoparticles. J Pharm Sci. 2012;101:233–44. doi:10.1002/jps.22763.
Prasanna RI, Anitha P, Chetty CM. Formulation and evaluation of bucco-adhesive tablets of sumatriptan succinate. Int J Pharm Investig. 2011;1:182–91. doi:10.4103/2230-973X.85971.
Perioli L, Pagano C. Preformulation studies of mucoadhesive tablets for carbamazepine sublingual administration. Colloids Surf B: Biointerfaces. 2013;102:915–22. doi:10.1016/j.colsurfb.2012.10.001.
Gavin A, Pham JT, Wang D, Brownlow B, Elbayoumi TA. Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. Int J Nanomedicine. 2015;10:1569–84. doi:10.2147/IJN.S75474.
Pendekaln MS, Tegginamat PK. Formulation and evaluation of a bioadhesive patch for buccal delivery of tizanidine. Acta Pharm Sin B. 2012;2:318–24. doi:10.1016/j.apsb.2011.12.012.
Hilgers AR, Conradi RA, Burton PS. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7:902–10. doi:10.1023/A:1015937605100.
van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1:175–85. doi:10.1517/17425255.1.2.175.
Chakraborti CK, Sahoo S, Behera PK. Role of different biodegradable polymers on the permeability of ciprofloxacin. J Adv Pharm Technol Res. 2014;5:140–6. doi:10.4103/2231-4040.137434.
Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials. 2014;35:7654–65. doi:10.1016/j.biomaterials.2014.05.053.
Acknowledgements
The authors gratefully acknowledge the National High Technology Research and Development Program of China (863 Program) (Project No. 2014AA022205) and Fundamental Research Funds for the Central Universities (No. 09ykpy67) for their financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 291 kb)
Rights and permissions
About this article
Cite this article
Yin, L., Wang, Y., Wang, C. et al. Nano-reservoir Bioadhesive Tablets Enhance Protein Drug Permeability Across the Small Intestine. AAPS PharmSciTech 18, 2329–2335 (2017). https://doi.org/10.1208/s12249-016-0709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0709-6