ABSTRACT
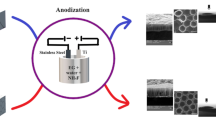
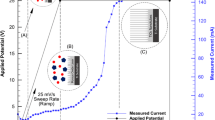
Titanium (Ti)-based materials is the most appropriate choices for the applications as orthopedic and dental implants. In this regard, ultrafine-grained (UFG) titanium with an enhanced mechanical properties and surface energy has attracted more attention. Titanium dioxide (TiO2) nanotubes grown on the titanium could enhance bone bonding, cellular response and are good reservoirs for loading drugs and antibacterial agents. This article investigates gentamicin loading into and release from the TiO2 nanotubes, grown on the UFG compared to coarse-grained (CG) titanium substrate surfaces. Equal Channel Angular Pressing (ECAP) was employed to produce the UFG structure titanium. TiO2 nanotubes were grown by the anodizing technique on both UFG and CG titanium substrate surfaces. Scanning electron microscopy (SEM) imaging confirmed TiO2 nanotube growth on the surface. The UV-vis spectroscopy analysis results show that the amount of gentamicin load-release in the anodized UFG titanium sample is higher than that of CG one which can be explained in terms of thicker TiO2 nanotube arrays layer formed on UFG sample. Moreover, the anodized UFG titanium samples released the drug in a longer time than CG (1 day for the UFG titanium vs. 3 h for the CG one). Regarding wettability analysis, anodized UFG titanium sample showed more enhanced hydrophilicity than CG counterpart. Therefore, the significantly smaller grain size of pure titanium provided by the ECAP technique coupled with appropriate subsequent anodization treatment not only offers a good combination of biocompatibility and adequate mechanical properties but also it provides a delayed release condition for gentamicin.





Similar content being viewed by others
REFERENCES
Nair M, Elizabeth E. Applications of titania nanotubes in bone biology. J Nanosci Nanotechnol. 2015;15(2):939–55.
Hajizadeh K, Ghobadi Alamdari S, Eghbali B. Stored energy and recrystallization kinetics of ultrafine grained titanium processed by severe plastic deformation. Phys B Condens Matter. 2013;417:33–8.
Maleki-Ghaleh H, Hajizadeh K, Hadjizadeh A, Shakeri MS, Ghobadi Alamdari S, Masoudfar S, et al. Electrochemical and cellular behavior of ultrafine-grained titanium in vitro. Mater Sci Eng C, Mater Biol Appl. 2014;39:299–304.
Mei S, Wang H, Wang W, Tong L, Pan H, Ruan C, et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35(14):4255–65.
Cipriano AF, Miller C, Liu H. Anodic growth and biomedical applications of TiO2 nanotubes. J Biomed Nanotechnol. 2014;10(10):2977–3003.
Yu W, Qian C, Jiang X, Zhang F, Weng W. Mechanisms of stem cell osteogenic differentiation on TiO2 nanotubes. Colloids Surf B: Biointerfaces. 2015;136:779–85.
Wu J, Li J, Wu F, He Z, Yang P, Huang N. Effect of micropatterned TiO2 nanotubes thin film on the deposition of endothelial extracellular matrix: for the purpose of enhancing surface biocompatibility. Biointerphases. 2015;10(4):04A302.
Yeniyol S, He Z, Yuksel B, Boylan RJ, Urgen M, Ozdemir T, et al. Antibacterial activity of as-annealed TiO2 nanotubes doped with Ag nanoparticles against periodontal pathogens. Bioinorg Chem Appl. 2014;2014:829496.
Xu Z, Lai Y, Wu D, Huang W, Huang S, Zhou L, et al. Antibacterial effects and biocompatibility of titania nanotubes with octenidine dihydrochloride/poly(lactic-co-glycolic acid). Biomed Res Int. 2015;2015:836939.
Yang Y, Ao HY, Yang SB, Wang YG, Lin WT, Yu ZF, et al. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int J Nanomedicine. 2016;11:2223–34.
Cui CX, Gao X, Qi YM, Liu SJ, Sun JB. Microstructure and antibacterial property of in situ TiO(2) nanotube layers/titanium biocomposites. J Mech Behav Biomed Mater. 2012;8:178–83.
Caliskan N, Bayram C, Erdal E, Karahaliloglu Z, Denkbas EB. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater Sci Eng C, Mater Biol Appl. 2014;35:100–5.
Shokuhfar T, Sinha-Ray S, Sukotjo C, Yarin AL. Intercalation of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2011;3:17380–6.
Hajizadeha K, Eghbali B, Topolskib K, Kurzydlowskib KJ. Ultra-fine grained bulk CP-Ti processed by multi-pass ECAP a warm deformation region. Mater Chem Phys. 2014;143(3):1032–8.
Hajizadeh K, Hadjizadeh A, Hashemi Nemati S. Titanium oxide nanotube formation and characterization on ultrafine-grained and coarse grained titanium surface by anodization submitted. 2016.
Jia H, Kerr LL. Sustained ibuprofen release using composite poly(lactic-co-glycolic acid)/titanium dioxide nanotubes from Ti implant surface. J Pharm Sci. 2013;102(7):2341–8.
Zhao J, Wang X, Sun T, Li L. Crystal phase transition and properties of titanium oxide nanotube arrays prepared by anodization. J Alloys Compd. 2007;434-435:792–5.
Hadjizadeh A, Ajji A, Jolicoeur M, Liberelle B, De Crescenzo G. Effects of electrospun nanostructure versus microstructure on human aortic endothelial cell behavior. J Biomed Nanotechnol. 2013;9(7):1195–209.
Dagil R, O’Shea C, Nykjaer A, Bonvin AM, Kragelund BB. Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the NMR structure of the 10th complement type repeat domain alone and in complex with gentamicin. J Biol Chem. 2013;288(6):4424–35.
ACKNOWLEDGEMENTS
Authors greatly acknowledge Dr. Saeed Hajizadeh (Maxillofacial surgeon and professor at the Shahid Beheshti University) for his valuable comments in conducting this research. The authors also would like to acknowledge the financial support of Iran National Science Foundation (INSF) and Iran Nanotechnology Initiative Council (INIC) for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemati, S.H., Hadjizadeh, A. Gentamicin-Eluting Titanium Dioxide Nanotubes Grown on the Ultrafine-Grained Titanium. AAPS PharmSciTech 18, 2180–2187 (2017). https://doi.org/10.1208/s12249-016-0679-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0679-8