Abstract
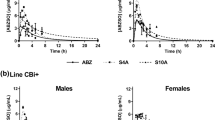
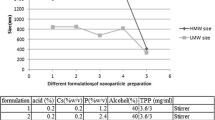
The oral route has notable advantages to administering dosage forms. One of the most important questions to solve is the poor solubility of most drugs which produces low bioavailability and delivery problems, a major challenge for the pharmaceutical industry. Albendazole is a benzimidazole carbamate extensively used in oral chemotherapy against intestinal parasites, due to its extended spectrum activity and low cost. Nevertheless, the main disadvantage is the poor bioavailability due to its very low solubility in water. The main objective of this study was to prepare microcrystal formulations by the bottom-up technology to increase albendazole dissolution rate, in order to enhance its antiparasitic activity. Thus, 20 novel microstructures based on chitosan, cellulose derivatives, and poloxamer as a surfactant were produced and characterized by their physicochemical properties and in vitro biological activity. To determine the significance of type and concentration of polymer, and presence or absence of surfactant in the crystals, the variables area under the curve, albendazole microcrystal solubility, and drug released (%) at 30 min were analyzed with a three-way ANOVA. This analysis indicated that the microcrystals made with hydroxyethylcellulose or chitosan appear to be the best options to optimize oral absorption of the active pharmaceutical ingredient. The in vitro evaluation of anthelmintic activity on adult forms of Trichinella spiralis identified system S10A as the most effective, of choice for testing therapeutic efficacy in vivo.







Similar content being viewed by others
References
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66.
Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev. 2016;103:105–20.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14(4):703–13.
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78–99.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60.
Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–49.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Salazar J, Ghanem A, Müller RH, Möschwitzer JP. Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur J Pharm Biopharm. 2012;81(1):82–90.
Kakran M, Shegokar R, Sahoo NG, Al Shaal L, Li L, Müller RH. Fabrication of quercetin nanocrystals: comparison of different methods. Eur J Pharm Biopharm. 2012;80(1):113–21.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Yang L, Chu D, Wang L, Ge G, Sun H. Facile synthesis of porous flower-like SrCO3 nanostructures by integrating bottom-up and top-down routes. Mater Lett. 2016;167:4–8.
Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–41.
Hu X, Chen X, Zhang L, Lin X, Zhang Y, Tang X, et al. A combined bottom–up/top–down approach to prepare a sterile injectable nanosuspension. Int J Pharm. 2014;472(1–2):130–9.
Paredes AJ, Llabot JM, Sánchez Bruni S, Allemandi D, Palma SD. Self-dispersible nanocrystals of albendazole produced by high pressure homogenization and spray-drying. Drug Dev Ind Pharm. 2016;42(10):1564–70.
Yallapu MM, Nagesh PKB, Jaggi M, Chauhan SC. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17(6):1341–56.
de Waard H, Frijlink HW, Hinrichs WLJ. Bottom-up preparation techniques for nanocrystals of lipophilic drugs. Pharm Res. 2011;28(5):1220–3.
Zhang Y, Hu X, Liu X, Dandan Y, Di D, Yin T, et al. Dry state microcrystals stabilized by an HPMC film to improve the bioavailability of andrographolide. Int J Pharm. 2015;493(1–2):214–23.
Tu L, Yi Y, Wu W, Hu F, Hu K, Feng J. Effects of particle size on the pharmacokinetics of puerarin nanocrystals and microcrystals after oral administration to rat. Int J Pharm. 2013;458(1):135–40.
Elsayed A, Al-Remawi M, Qinna N, Farouk A, Al-Sou’od KA, Badwan AA. Chitosan–sodium lauryl sulfate nanoparticles as a carrier system for the in vivo delivery of oral insulin. AAPS PharmSciTech. 2011;12(3):958–64.
Cervera MF, Heinämäki J, de la Paz N, López O, Maunu SL, Virtanen T, et al. Effects of spray drying on physicochemical properties of chitosan acid salts. AAPS PharmSciTech. 2011;12(2):637–49.
Sezer AD, Hatipoglu F, Cevher E, Oğurtan Z, Bas AL, Akbuğa J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2007;8(2):94–101.
Higueras L, López-Carballo G, Cerisuelo JP, Gavara R, Hernández-Muñoz P. Preparation and characterization of chitosan/HP-β-cyclodextrins composites with high sorption capacity for carvacrol. Carbohydr Polym. 2013;97(2):262–8.
Amin M, Abbas NS, Hussain MA, Edgar KJ, Tahir MN, Tremel W, et al. Cellulose ether derivatives: a new platform for prodrug formation of fluoroquinolone antibiotics. Cellulose. 2015;22(3):2011–22.
Regdon G, Hegyesi D, Pintye-Hódi K. Thermal study of ethyl cellulose coating films used for modified release (MR) dosage forms. J Therm Anal Calorim. 2012;108(1):347–52.
Baltzley S, Mohammad A, Malkawi AH, Al-Ghananeem AM. Intranasal drug delivery of olanzapine-loaded chitosan nanoparticles. AAPS PharmSciTech. 2014;15(6):1598–602.
Charoenthai N, Kleinebudde P, Puttipipatkhachorn S. Influence of chitosan type on the properties of extruded pellets with low amount of microcrystalline cellulose. AAPS PharmSciTech. 2007;8(3):99–109.
Dubey RR, Parikh RH. Two-stage optimization process for formulation of chitosan microspheres. AAPS PharmSciTech. 2009;5(1):20–8.
Piyakulawat P, Praphairaksit N, Chantarasiri N, Muangsin N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS PharmSciTech. 2007;8(4):120–30.
Wang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14(2):585–92.
Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86(2–3):141–59.
Daniel-Mwambete K, Torrado S, Cuesta-Bandera C, Ponce-Gordo F, Torrado JJ. The effect of solubilization on the oral bioavailability of three benzimidazole carbamate drugs. Int J Pharm. 2004;272(1–2):29–36.
United States Pharmacopeia, USP. United States pharmacopeia and national formulary convention. Rockville (MD); 2009.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27(1):48–9.
García A, Leonardi D, Piccirilli GN, Mamprin ME, Olivieri AC, Lamas MC. Spray drying formulation of albendazole microspheres by experimental design. In vitro–in vivo studies. Drug Dev Ind Pharm. 2015;41(2):244–52.
Dražić S, Sladoje N, Lindblad J. Estimation of Feret’s diameter from pixel coverage representation of a shape. Pattern Recogn Lett. 2016;80:37–45.
Vasconi MD, Bertorini G, Codina AV, Indelman P, Masso RJD, Hinrichsen LI. Phenotypic characterization of the response to infection with Trichinella spiralis in genetically defined mouse lines of the CBi-IGE stock. Open J Vet Med. 2015;5(5):12.
Bikiaris DN. Solid dispersions, part I: recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2011;8(11):1501–19.
Codina AV, García A, Leonardi D, Vasconi MD, Di Masso RJ, Lamas MC, et al. Efficacy of albendazole:β-cyclodextrin citrate in the parenteral stage of Trichinella spiralis infection. Int J Biol Macromol. 2015;77:203–6.
Acknowledgments
J.P. is grateful to CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) for a doctoral fellowship. A.V.C is grateful to CIUNR (Consejo de Investigaciones, Universidad Nacional de Rosario) for a research fellowship.
This work was supported by the Universidad Nacional de Rosario, Consejo Nacional de Investigaciones Científicas y Técnicas (Project N° PIP 112-201001-00194) and Agencia Nacional de Promoción Científica y Tecnológica (Project N° PICT 2006-1126).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guest Editors: Claudio Salomon, Francisco Goycoolea, and Bruno Moerschbacher
Josefina Priotti and Ana V. Codina contributed equally to this work.
Rights and permissions
About this article
Cite this article
Priotti, J., Codina, A.V., Leonardi, D. et al. Albendazole Microcrystal Formulations Based on Chitosan and Cellulose Derivatives: Physicochemical Characterization and In Vitro Parasiticidal Activity in Trichinella spiralis Adult Worms. AAPS PharmSciTech 18, 947–956 (2017). https://doi.org/10.1208/s12249-016-0659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0659-z