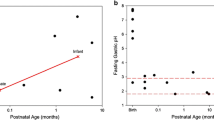
Abstract
Despite many documented differences in gut physiology compared to humans, the beagle dog has been successfully used as a preclinical model for assessing the relative bioavailability of dosage forms during formulation development. However, differences in pH and bile salt concentration and micellar structure between dog and human intestinal fluids may influence the solubility and dissolution behavior of especially BCS II/IV compounds. Recently, a canine fasted simulated intestinal fluid (FaSSIFc) mimicking the composition in the lumen of the beagle dog under the fasted state has been proposed. In this manuscript, we present the utilization of FaSSIFc to compare solubility of several preclinical candidates against human FaSSIF. While solubility of free bases and neutral compounds was easily predicted by the relative amounts of sodium taurocholate in the fluids, free acids were shown to be much more soluble in FaSSIFc owing to both the solubility at higher pH as well as the increased bile salt concentration. For one of the model compounds, we demonstrate that the high solubility necessitates the need for a formulation comparison at a relatively higher dose in the dog to mimic the outcome of a human relative bioavailability study. Finally, we show how using the solubility value in FaSSIFc for the same compound results in better predictability of the plasma concentration profiles in dogs from a physiologically based absorption model. The collective data indicate that caution and more detailed measurements are required if the dog is used as the preclinical model for the development of formulations of weak acids.




Similar content being viewed by others
References
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
Galia E, Nicolaides E, Horter D, Lobenberg R, Reppas C, Dressman J. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Vertzoni M, Fotaki N, Nicolaides E, Reppas C, Kostewicz E, Stippler E, et al. Dissolution media simulating the intralumenal composition of the small intestine: physiological issues and practical aspects. J Pharm Pharmacol. 2004;56(4):453–62.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66.
Wu Y, Kesisoglou F. Screening immediate release oral formulations in pharmaceutical industry. In: Dressman J, Reppas C, editors. Oral drug absorption: prediction and assessment. Zug: Informa Healthcare; 2010.
Heikkinen AT, Fowler S, Gray L, Li J, Peng Y, Yadava P, et al. In vitro to in vivo extrapolation and physiologically based modeling of cytochrome p450 mediated metabolism in beagle dog gut wall and liver. Mol Pharm. 2013;10(4):1388–99.
Dressman JB. Comparison of canine and human gastrointestinal physiology. Pharm Res. 1986;3(3):123–31.
Augustijns P, Wuyts B, Hens B, Annaert P, Butler J, Brouwers J. A review of drug solubility in human intestinal fluids: implications for the prediction of oral absorption. Eur J Pharm Sci. 2014;57:322–32.
Arndt M, Chokshi H, Tang K, Parrott NJ, Reppas C, Dressman JB. Dissolution media simulating the proximal canine gastrointestinal tract in the fasted state. Eur J Pharm Biopharm. 2013;84(3):633–41.
Kesisoglou F. Use of preclinical dog studies and absorption modeling to facilitate late stage formulation bridging for a BCS II drug candidate. AAPS Pharmscitech. 2014;15(1):20–8.
Parrott N, Lukacova V, Fraczkiewicz G, Bolger M. Predicting pharmacokinetics of drugs using physiologically based modeling-application to food effects. AAPS J. 2009;11(1):45–53.
Papich MG, Martinez MN. Applying biopharmaceutical classification system (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J. 2015:1–17.
http://biorelevant.com. FaSSIF, FeSSIF & FaSSGF powder—how to make FaSSIF, FeSSIF & FaSSGF dissolution media easily. 2015 [cited 2015 Feb 4, 2015]; Available from: http://biorelevant.com/fassif-fessif-fassgf-dissolution-media/fasted-fed-state-simulated-intestinal-gastric-fluid/how-to-make/
Kloefer B, van Hoogevest P, Moloney R, Kuentz M, Leigh MLS, Dressman J. Study of a standardized taurocholate—lecithin powder for preparing the biorelevant media FeSSIF and FaSSIF. Dissolution Technol. 2010;17(3):6–13.
Rippie EG, Lamb DJ, Romig PW. Solubilization of weakly acidic and basic drugs by aqueous solutions of polysorbate 80. J Pharm Sci. 1964;53:1346–8.
Jinno J, Oh D, Crison JR, Amidon GL. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J Pharm Sci. 2000;89(2):268–74.
Sheng JJ, Kasim NA, Chandrasekharan R, Amidon GL. Solubilization and dissolution of insoluble weak acid, ketoprofen: effects of pH combined with surfactant. Eur J Pharm Sci. 2006;29(3–4):306–14.
Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012;56(6):3020–6.
Acknowledgments
The authors would like to thank Lan Jin, Kim Manser, and Becky Nissley for their support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
All animal study protocols were reviewed and approved by the Merck IACUC (Institutional Animal Care and Use Committee). The Guide and Animal Welfare regulations were followed in the conduct of the animal studies. Veterinary care was given to any animals requiring medical attention.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Walsh, P.L., Stellabott, J., Nofsinger, R. et al. Comparing Dog and Human Intestinal Fluids: Implications on Solubility and Biopharmaceutical Risk Assessment. AAPS PharmSciTech 18, 1408–1416 (2017). https://doi.org/10.1208/s12249-016-0611-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0611-2