Abstract
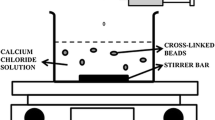
External ionotropic gelation offers a unique possibility to entrap multivalent ions in a polymer structure. The aim of this experimental study was to prepare new drug-free sodium alginate (ALG) particles cross-linked by Cu2+ ions and to investigate their technological parameters (particle size, sphericity, surface topology, swelling capacity, copper content, release of Cu2+ ions, mucoadhesivity) and biological activity (cytotoxicity and efficiency against the most common vaginal pathogens—Herpes simplex, Escherichia coli, Candida albicans) with respect to potential vaginal administration. Beads prepared from NaALG dispersions (3 or 4%) were cross-linked by Cu2+ ions (0.5 or 1.0 M CuCl2) using external ionotropic gelation. Prepared mucoadhesive beads with particle size over 1000 μm exhibited sufficient sphericity (all ˃0.89) and copper content (214.8–249.07 g/kg), which increased with concentration of polymer and hardening solution. Dissolution behaviour was characterized by extended burst effect, followed by 2 h of copper release. The efficiency of all samples against the most common vaginal pathogens was observed at cytotoxic Cu2+ concentrations. Anti-HSV activity was demonstrated at a Cu2+ concentration of 546 mg/L. Antibacterial activity of beads (expressed as minimum inhibition concentration, MIC) was influenced mainly by the rate of Cu2+ release which was controlled by the extent of swelling capacity. Lower MIC values were found for E. coli in comparison with C. albicans. Sample ALG-3_1.0 exhibited the fastest copper release and was proved to be the most effective against both bacteria. This could be a result of its lower polymer concentration in combination with smaller particle size and thus larger surface area.









Similar content being viewed by others
References
Arredondo M, Núñez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26(4-5):313–27. doi:10.1016/j.mam.2005.07.010.
Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. 2005;26(4-5):235–44. doi:10.1016/j.mam.2005.07.013.
Grillo CA, Reigosa MA, de Mele Fernández Lorenzo M. Effects of copper ions released from metallic copper on CHO-K1 cells. Mutat Res-Gen Tox En. 2009;672(1):45–50. doi:10.1016/j.mrgentox.2008.09.012.
Cai X, Zhang B, Liang Y, Zhang J, Yan Y, Chen X, et al. Study on the antibacterial mechanism of copper ion- and neodymium ion-modified α-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloid Surf B. 2015;132:281–9. doi:10.1016/j.colsurfb.2015.05.027.
Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Ag. 2009;33(6):587–90. doi:10.1016/j.ijantimicag.2008.12.004.
Gabbay J, Mishal J, Magen E, Zatcoff R, Shemer-Avni Y, Borkow GJ. Copper oxide impregnated textiles with potent biocidal activities. Ind Text. 2006;35:323–35. doi:10.1177/1528083706060785.
Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89(11):1175–86. doi:10.1177/0022034510377794.
Roblero L, Guadarrama A, Lopez T, Zegers-Hochschild F. Effect of copper ion on the motility, viability, acrosome reaction and fertilizing capacity of human spermatozoa in vitro. Reprod Fert Develop. 1996;8(5):871–4. doi:10.1071/RD9960871.
Borkow G, Gabbay J. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 2004;18(14):1728–30. doi:10.1096/fj.04-2029fje.
Sagripanti JL, Routson LB, Lytle CD. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microb. 1993;59(12):4374–6.
Sagripanti JL, Routson LB, Bonifacino AC, Lytle CD. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob Agents Ch. 1997;41(4):812–7.
Sagripanti JL, Lightfoote MM. Cupric and ferric ions inactivate HIV. AIDS Res Hum Retrov. 1996;12(4):333–7. doi:10.1089/aid.1996.12.333.
Funami T, Fang Y, Noda S, Ishihara S, Nakauma M, Draget KI, et al. Rheological properties of sodium alginate in an aqueous system during gelation in relation to supermolecular structures and Ca2+ binding. Food Hydrocolloid. 2009;23(7):1746–55. doi:10.1016/j.foodhyd.2009.02.014.
Goh CH, Heng PWS, Chan LW. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohyd Polym. 2012;88(1):1–12. doi:10.1016/j.carbpol.2011.11.012.
Laurienzo P. Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs. 2010;8(9):2435–65. doi:10.3390/md8092435.
Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliver Rev. 1998;31(3):267–85. doi:10.1016/j.addr.2012.09.007.
Tønnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28(6):621–30. doi:10.1081/DDC-120003853.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26. doi:10.1016/j.progpolymsci.2011.06.003.
Khotimchenko YS, Kovalev VV, Savchenko OV, Ziganshina OA. Physical-chemical properties, physiological activity, and usage of alginates, the polysaccharides of brown algae. Russ J Mar Biol. 2001;27(1):853–64.
Sharma CP. Chapter 9. 2. 3. In: Grøndhal L, Lawrie G, Jejurikar A, editors. Biointegration of medical implant materials: science and design, vol. 1. Cambridge: Woodhead Publishing Limited; 2010. p. 239.
Palmeira-de-Oliveira R, Palmeira-de-Oliveira A, Martinez-de-Oliveira J. New strategies for local treatment of vaginal infections. Adv Drug Deliver Rev. 2015;92:105–22. doi:10.1016/j.addr.2015.06.008.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliver Rev. 2009;61(2):86–100. doi:10.1016/j.addr.2008.09.012.
Ensign LM, Hoen TE, Maisel K, Cone RA, Hanes JS. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials. 2013;34(28):6922–9. doi:10.1016/j.biomaterials.2013.05.039.
Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103(2):301–13. doi:10.1016/j.jconrel.2004.11.034.
Smýkalová I, Horáček J, Hýbl M, Bjelková M, Pavelek M, Krulikovská T, et al. Seed type identification by image analysis—correlation of nutrients with size, shape and colour characteristics of seeds. Chem Listy. 2011;105(2):138–45.
Rabišková M, Häring A, Minczingerová K, Havlásek M, Musilová P. Microcrystalline cellulose in oral dosage forms. Chem Listy. 2007;101(1):70–7.
Lin W-C, Yu D-G, Yang M-C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: swelling kinetics and drug delivery properties. Colloid Surface B. 2005;44(2-3):143–51. doi:10.1016/j.colsurfb.2005.06.010.
European Pharmacopoeia Commission et al. European Pharmacopoeia 8th Edition: Supplement 8.0, 4.1.3. Buffer solutions. Strasbourg: Council of Europe; 2014.
Dai Y-N, Li P, Zhang J-P, Wang A-Q, Wei Q. Swelling characteristics and drug delivery properties of nifedipine-loaded pH sensitive alginate–chitosan hydrogel beads. J Biomed Mater Res B. 2008;86B(2):493–500. doi:10.1002/jbm.b.31046.
Neves J, Amaral MH, Bahia MF. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: influence of different testing conditions and instrumental parameters. Eur J Pharm Biopharm. 2008;69(2):622–32. doi:10.1016/j.ejpb.2007.12.007.
Hiorth M, Nilsen S, Tho I. Bioadhesive mini-tablets for vaginal drug delivery. Pharmaceutics. 2014;6(3):494–511. doi:10.3390/pharmaceutics6030494.
Wu J, Wang L, He J, Zhu C. In vitro cytotoxicity of Cu2+, Zn2+, Ag+ and their mixtures on primary human endometrial epithelial cells. Contraception. 2012;85(5):509–18. doi:10.1016/j.contraception.2011.09.016.
Lupo B, Maestro A, Gutiérrez JM, Gonzalez C. Characterization of alginate beads with encapsulated cocoa extract to prepare functional food: comparison of two gelation mechanisms. Food Hydrocolloid. 2015;49:25–34. doi:10.1016/j.foodhyd.2015.02.023.
Puguan JMC, Yu X, Kim HJ. Characterization of structure, physico-chemical properties and diffusion behavior of Ca-alginate gel beads prepared by different gelation methods. Colloid Interf Sci. 2014;432:109–16. doi:10.1016/j.jcis.2014.06.048.
Chan LW, Lee HY, Heng PWS. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohyd Polym. 2006;63(2):176–87. doi:10.1016/j.carbpol.2005.07.033.
Soni ML, Kumar M, Namdeo KP. Sodium alginate microspheres for extending drug release: formulation and in vitro evaluation. Int J Drug Delivery. 2010;2:64–8. doi:10.5138/ijdd.2010.0975.0215.02013.
Smrdel P, Bogataj M, Mrhar A. The influence of selected parameters on the size and shape of alginate beads prepared by ionotropic gelation. Sci Pharm. 2008;76:77–89. doi:10.3797/scipharm.0611-07.
Manjanna KM, Shivakumar B, Raichur AM, Kumar TMP. Formulation and characterization of dexibuprofen microbeads by microemulsificaton-ionotropic gelation technique. Int J Drug Formul Res. 2011;2:120–38.
Sathali AAH, Varun J. Formulation, development and in vitro evaluation of candesartan cilexetil mucoadhesive microbeads. Int J Curr Pharm Res. 2012;4(3):109–18.
Focaroli S, Teti G, Salvatore V, Orienti I, Falconi M. Calcium/cobalt alginate beads as functional scaffolds for cartilage tissue engineering. Stem Cells Int. 2016;2016:2030478. doi:10.1155/2016/2030478.
Klokk TI, Melvik JE. Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul. 2002;19(4):415–24. doi:10.1080/02652040210144234.
Lee B-B, Ravindra P, Chan E-S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem Eng Technol. 2013;36(10):1627–42. doi:10.1002/ceat.201300230.
Deasy PB, Law MFL. Use of extrusion-spheronization to develop an improved oral dosage form of indomethacin. Int J. 1997;148(2):201–9. doi:10.1016/S0378-5173(96)04846-6.
Manjanna KM, Shivakumar B, Pramod Kumar TM. Diclofenac sodium microbeads for oral sustained drug delivery. Int J PharmTech Res. 2009;1:317–27.
Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 2002;249(1-2):165–74. doi:10.1016/S0378-5173(02)00487-8.
Bajpai SK, Tankhiwale R. Investigation of water uptake behavior and stability of calcium alginate/chitosan bi-polymeric beads: part-1. React Funct Polym. 2006;66(6):645–58. doi:10.1016/j.reactfunctpolym.2005.10.017.
Hassan R, Tirkistani F, Zaafarany I, Fawzy A, Khairy M, Iqbal S. Polymeric biomaterial hydrogels. I. Behavior of some ionotropic cross-linked metal-alginate hydrogels especially copper-alginate membranes in some organic solvents and buffer solutions. Adv Biosci Biotechnol. 2012;3(7):845–54. doi:10.4236/abb.2012.37105.
Hassan RM, El-Shatoury SA, Mousa MA, Hassan A. Kinetics and mechanism of sol-gel transformation for polyelectrolytes of capillary copper alginate ionotropic membranes. Eur Polym J. 1988;24:1173–5. doi:10.1016/0014-3057(88)90106-1.
Machida-Sano I, Ogawa S, Ueda H, Kimura Y, Satoh N, Namiki H. Effects of composition of iron-cross-linked alginate hydrogels for cultivation of human dermal fibroblasts. Int J Biomater. 2012;2012:820513. doi:10.1155/2012/820513.
Palmer C, Guerinot ML. A question of balance: facing the challenges of Cu, Fe and Zn homeostasis. Nat Chem Biol. 2009;5(5):333–40. doi:10.1038/nchemboi.166.
Fang Y, Al-Assaf S, Phillips GO, Nishinari K, Funami T, Williams PA, et al. Multiple steps and critical behaviors of the binding of calcium to alginate. J Phys Chem B. 2007;111:2456–62.
Von Burkersroda F, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23(21):4221–31. doi:10.1016/S0142-9612(02)00170-9.
Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. doi:10.1016/S0939-6411(03)00161-9.
Khazaeli P, Pardakhty A, Hassanzadeh F. Formulation of ibuprofen beads by ionotropic gelation. Iran J Pharm Res. 2008;7(3):163–70.
Yang S, Washington C. Drug release from microparticulate systems. In: Benita S, editor. Microencapsulation: methods and industrial applications. 2nd ed. Boca Raton: Taylor & Francis Group, LLC; 2006. p. 183–212.
Velings NM, Mestdagh MM. Physico-chemical properties of alginate gel beads. Polym Gels Netw. 1995;3:311–30.
Tavakol M, Vasheghani-Farahani E, Hashemi-Najafabadi S. The effect of polymer and CaCl2 concentrations on the sulfasalazine release from alginate-N,O-carboxymethyl chitosan beads. Prog Biomater. 2013;2:10. http://www.progressbiomaterials.com/content/2/1/10.
Caramella CM, Rossi S, Ferrari F, Bonferoni MC, Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv Drug Deliver Rev. 2015;92:39–52. doi:10.1016/j.addr.2015.02.001.
Sagripanti J-L, Goering PL, Lamanna A. Interaction of copper with DNA and antagonism by other metals. Toxicol Appl Pharmacol. 1991;110(3):477–85. doi:10.1016/0041-008X(91)90048-J.
Magaῆa SM, Quintana P, Aguilar DH, Toledo JA, Ángeles-Chávez C, Cortés MA, et al. Antibacterial activity of montmorillonites modified with silver. J Mol Catal A-Chem. 2008;281(1-2):192–9. doi:10.1016/j.molcata.2007.10.024.
Kejdušová M, Vysloužil J, Kubová K, Celer V, Krásná M, Pechová A, et al. Antimicrobial properties of microparticles based on carmellose cross-linked by Cu2+ ions. Biomed Res Int. 2015;2015:790720. doi:10.1155/2015/790720.
Zhao J, Feng HJ, Tang HQ, Zheng JH. Bactericidal and corrosive properties of silver implanted TiN thin films coated on AISI317 stainless steel. Surf Coat Tech. 2007;201(9-11):5676–9. doi:10.1016/j.surfcoat.2006.07.172.
Martínez Medina JJ, Islas MS, López Tévez LL, Ferrer EG, Okulik NB, Williams PAM. Copper(II) complexes with cyanoguanidine and o-phenanthroline: theoretical studies, in vitro antimicrobial activity and alkaline phosphatase inhibitory effect. J Mol Struct. 2014;1058:298–307. doi:10.1016/j.molstruc.2013.11.014.
Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microb. 2011;77(5):1541–7. doi:10.1128/AEM.02766-10.
Tong G, Yulong M, Peng G, Zirong X. Antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis. Vet Microbiol. 2005;105(2):113–22. doi:10.1016/j.vetmic.2004.11.003.
Dan YG, Ni HW, Xu BF. Microstructure and antibacterial properties of AISI 420 stainless steel implanted by copper ions. Thin Solid Films. 2005;492(1-2):93–100. doi:10.1016/j.tsf.2005.06.100.
Hu CH, Xia MS. Adsorption and antibacterial effect of copper exchanged montmorillonite on Escherichia coli K88. Appl Clay Sci. 2006;31(3-4):180–4. doi:10.1016/j.clay.2005.10.010.
Koch AL. Growth and form of the bacterial cell wall. Am Sci. 1990;78:327–40.
Weissman Z, Berdicevsky I, Cavari B-Z, Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci U S A. 2000;97(7):3520–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
This experimental work was realized by support IGA VFU Brno Czech Republic, project 306/2015/FaF.
Rights and permissions
About this article
Cite this article
Pavelková, M., Kubová, K., Vysloužil, J. et al. Biological Effects of Drug-Free Alginate Beads Cross-Linked by Copper Ions Prepared Using External Ionotropic Gelation. AAPS PharmSciTech 18, 1343–1354 (2017). https://doi.org/10.1208/s12249-016-0601-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0601-4