ABSTRACT
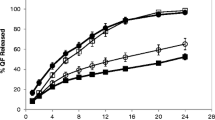
The solubility of weakly basic drugs in passage through gastrointestinal tract leads to their pH-dependent release from extended release formulations and to lower drug absorption and bioavailability. The aim of this study was to modulate the micro-environmental pH of hypromellose/montanglycol wax matrices and to observe its influence on the release of weakly basic drug verapamil hydrochloride (VH) with a pH-dependent solubility with respect to gel layer formation and its dynamics. For this study, malic and succinic acids differing in their solubility and pKa were selected as pH modifiers. The dissolution studies were performed by the method of changing pH. Within the same conditions, pH, thickness, and penetration force of the gel layer were measured as well. From the PCA sub-model, it is evident that a higher acid concentration ensured lower gel pH and conditions for higher drug solubility, thus creating larger gel layer with smaller rigidity, resulting in higher VH release during the dissolution test. Incorporation of stronger and more soluble malic acid (100 mg/tablet) created the most acidic and the thickest gel layer through which a total of 74% of VH was released. Despite having lower strength and solubility, matrices containing succinic acid (100 mg/tablet) released a comparable 71% of VH in a manner close to zero-order kinetics. The thinner and less rigid gel layers of the succinic acid matrices allowed an even slightly faster VH release at pH 6.8 than from matrices containing malic acid. Thus acid solubility is more parametrically significant than acid pKa for drug release at pH 6.8.









Similar content being viewed by others
REFERENCES
Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 2001;46:75–87.
Jambhekar SS, Breen PJ. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov Today. 2013;18(23–24):1173–84.
Fahr A, Liu X. Drug delivery strategies for poorly watersoluble drugs. Expert Opin Drug Deliv. 2007;4:403–16.
Govindarajan R, Nagarsenker MS. Basic drug enterosoluble polymer coevaporates: development of oral controlled release systems. Drug Dev Ind Pharm. 2004;30:847–57.
Gutsche S, Krause M, Kranz H. Strategies to overcome ph-dependent solubility of weakly basic drugs by using different types of alginates. Drug Dev Ind Pharm. 2008;34(12):1277–84.
Howard JR, Timmins P. Controlled release formulation. US Patent No. 1988;4:792–452.
Dashevskya A, Kolterb K, Bodmeier R. pH-independent release of a basic drug from pellets coated with the extended release polymer dispersion Kollicoatw SR 30 D and the enteric polymer dispersion Kollicoatw MAE 30 DP. Eur J Pharm Biopharm. 2004;58:45–9.
Tiwari SB, Rajabi-Siahboomi AR. Extended-Release Oral Drug Delivery Technologies: Monolithic Matrix Systems. In: Jain KK, editor. Drug Delivery Systems. Basel: Humana Press; 2008. p. 217–44.
Tatavarti AS, Hoag SW. Microenvironmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J Pharm Sci. 2006;95(7):1459–68.
Streubel A, Siepmann J, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Control Release. 2000;67:101–10.
Siepe S, Lueckel B, Kramer A, Ries A, Gurny R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int J Pharm. 2006;316:14–20.
Mehta DM, Parejiya PB, Barot BS, Shelat PK. Investigation of the drug release modulating effect of acidifiers in modified release oral formulation of cinnarizine. Asian J Pharm Sci. 2012;7(3):193–201.
Kranz H, Guthmann C, Wagner T, Lipp R, Reinhard J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Eur J Pharm Sci. 2005;26:47–53.
Espinoza R, Hong E, Villafuerte L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int J Pharm. 2000;201:165–73.
Nie S, Pan W, Li X, Wu X. The effect of citric acid added to hyroxypropylmethylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev Ind Pharm. 2004;30:627–35.
Bolourchian N, Dadashzadeh S. pH-independent release of propranolol hydrochloride from HPMC based matrices using organic acids. DARU. 2008;16(3):136–42.
Dvořáčková K, Doležel P, Mašková E, Muselík J, Kejdušová M, Vetchý D. The effect of acid ph modifiers on the release characteristics of weakly basic drug from hydrophilic-lipophilic matrices. AAPS PharmSciTech. 2013;14(4):1341–8.
Sateesha SB, Rajamma AJ, Narode MK, Vyas BD. Influence of organic acids on diltiazem hcl release kinetics from hydroxypropyl methyl cellulose matrix tablets. J Young Pharm. 2010;2(3):229–33.
Phadtare D, Phadtare GNB, Asawat M. Hypromellose—a choice of polymer in extended release tablet formulation. World J Pharm Pharm Sci. 2014;3:551–66.
Mu B, Thompson MR. Examining the mechanics of granulation with a hot melt binder in a twin-screw extruder. Chem Eng Sci. 2012;81:46–56.
Mašić I, Ilić I, Dreu R, Ibrić S, Parojčić J, Đurić Z. An investigation into the effect of formulation variables and process parameters on characteristics of granules obtained by in situ fluidized hot melt granulation. Int J Pharm. 2012;423(2):202–12.
Ahrabi SF, Madsen G, Dyrstad K, Sande SA, Graffner C. Development of pectin matrix tablets for colonic delivery of model drug ropivacaine. Eur J Pharm Sci. 2000;10(1):43–52.
Tabasi SH, Moolchandani V, Fahmy R, Hoag SW. Sustained release dosage forms dissolution behavior prediction: a study of matrix tablets using NIR spectroscopy. Int J Pharm. 2009;382(1):1–6.
Li W, Woldu A, Araba L, Winstead D. Determination of water penetration and drug concentration profiles in HPMC‐based matrix tablets by near infrared chemical imaging. J Pharm Sci. 2010;99(7):3081–8.
Wei X, Yuan CH, Zhang H, Li B. Montan wax: the state-of-the-art review. J Chem Pharm Res. 2014;6(6):1230–6.
Franc A, Sova P. Oral pharmaceutical composition for targeted transport of a platinum complex into the colorectal region, method for producing and use as medicament thereof European Patent Office, http://v3.espacenet.com, 20.1.2009.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Patil H, Tiwari RV, Upadhye SB, Vladyka RS, Repka MA. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process. Int J Pharm. 2015;496(1):33–41.
Pěček D, Štýbnarová M, Mašková E, Doležel P, Kejdušová M, Vetchý D, et al. The use of texture analysis in development and evaluation of matrix tablets with prolonged drug release. Chem Listy. 2014;108:483–7.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. https://www.r-project.org/.
Dawson RMC et al. Data for biochemical research, Oxford, Clarendon Press; 1959
Stahl PH, Wermuth CG. Handbook of pharmaceutical salts: properties, selection and use. Berlin: Wiley; 2002.
Dvořáčková K, Rabišková M, Masteiková R, Muselík J, Krejčová K. Soluble filler as the dissolution profile modulator for slightly soluble drugs in matrix tablets. Drug Dev Ind Pharm. 2009;35:930–40.
Healy AM, Corrigan OI. The influence of excipient particle size, solubility and acid strength on the dissolution of an acidic drug from two-component compacts. Int J Pharm. 1996;143:211–21.
Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. London: The Pharmaceutical Press; 2003.
Rao NGR, Hadi MA, Panchal H. Formulation and evaluation of biphasic drug delivery system of montelukast sodium for chronotherapy. Int J Pharm Chem Sci. 2012;1(3):1256–65.
Lopes CM, Lobo JMS, Pinto JF, Costa PC. Compressed matrix core tablet as a quick/slow dual-component delivery system containing ibuprofen. AAPS PharmSciTech. 2007;8(3):E195–202.
Akbari J, Nokhodchi A, Farid D, Adrangui M, Siahi-Shadbad MR, Saeedi M. Development and evaluation of buccoadhesive propranolol hydrochloride tablet formulations: effect of fillers. IL 540 Farmacol. 2004;59:155–61.
Ford JL, Rubinstein MH, Mc Caul F, Hogan JE, Edgar PE. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcelluose tablets. Int J Pharm. 1987;40:223–34.
Ranga Roa KV, Padmalatha Devi K, Buri P. Cellulose matrices for zero-order release of soluble drugs. Drug Dev Ind Pharm. 1988;14(15–17):2299–320.
Kim CJ. Effects of drug solubility, drug loading and polymer molecular weight on drug release from Polyox® tablets. Drug Dev Ind Pharm. 1998;24:645–51.
Velasco MV, Ford JL, Rowe P, Rajabi-Siahboomi AR. Influence of drug: hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J Control Release. 1999;57:75–85.
Zuleger S, Lippold BC. Polymer particle erosion controlling drug release. I. Factors influencing drug release and characterization of the release mechanism. Int J Pharm. 2001;217:139–52.
Efentakis M, Pagoni I, Vlachou M, Avgoustakis K. Dimensional changes, gel layer evolution and drug release studies in hydrophilic matrices loaded with drugs of different solubility. Int J Pharm. 2007;339:66–75.
Bettini R, Catellani PL, Santi P, Massimo G, Peppas NA, Colombo P. Translocation of drug particles in HPMC matrix gel layer: effect of drug solubility and influence on release rate. J Control Release. 2001;70:383–91.
Fuertes I, Caraballo I, Miranda A, Millán M. Study of critical points of drugs with different solubilities in hydrophilic matrices. Int J Pharm. 2010;383:138–46.
Varma MVS, Kaushal AM, Garg S. Influence of micro-environmental pH on the gel layer behavior and release of a basic drug from various hydrophilic matrices. J Control Release. 2005;103:499–510.
Yang L. Determination of continuous changes in the gel layer of poly(ethylene oxide) and HPMC tablets undergoing hydration: a texture analysis study. Pharm Res. 1998;15:1902–6.
Zuleger S, Fassihi R, Lippold BC. Polymer particle erosion controlling drug release (II): swelling investigations to clarify the release mechanism. Int J Pharm. 2002;247(1–2):23–37.
Varma MVS, Kaushal AM, Garg A, Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am J Drug Deliv. 2004;2(1):43–57.
Colombo P, Bettini R, Peppas NA. Observation of swelling process and diffusion front position during swelling in hydroxypropyl methyl cellulose (HPMC) matrices containing a soluble drug. J Control Release. 1999;61:83–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mašková, E., Kubová, K., Vysloužil, J. et al. Influence of pH Modulation on Dynamic Behavior of Gel Layer and Release of Weakly Basic Drug from HPMC/Wax Matrices, Controlled by Acidic Modifiers Evaluated by Multivariate Data Analysis. AAPS PharmSciTech 18, 1242–1253 (2017). https://doi.org/10.1208/s12249-016-0588-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0588-x