Abstract
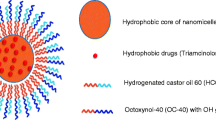
The purpose of this study was to develop a clear aqueous mixed nanomicellar formulation (MNF) of dexamethasone utilizing both d-α-tocopherol polyethylene glycol-1000 succinate (Vit E TPGS) and octoxynol-40 (Oc-40). In this study, Vit E TPGS and Oc-40 are independent variables. Formulations were prepared following solvent evaporation method. A three level full-factorial design was applied to optimize the formulation based on entrapment efficiency, size, and polydispersity index (PDI). A specific blend of Vit E TPGS and Oc-40 at a particular wt% ratio (4.5:2.0) produced excellent drug entrapment, loading, small mixed nanomicellar size and narrow PDI. Solubility of DEX in MNF is improved by ~6.3-fold relative to normal aqueous solubility. Critical micellar concentration (CMC) for blend of polymers (4.5:2.0) was found to be lower (0.012 wt%) than the individual polymers (Vit E TPGS (0.025 wt%) and Oc-40 (0.107 wt%)). No significant effect on mixed nanomicellar size and PDI with one-factor or multi-factor interactions was observed. Qualitative 1H NMR studies confirmed absence of free drug in the outer aqueous MNF medium. MNF appeared to be highly stable. Cytotoxicity studies on rabbit primary corneal epithelial cells did not indicate any toxicity suggesting MNF of dexamethasone is safe and suitable for human topical ocular drops after further in vivo evaluations.









Similar content being viewed by others
REFERENCES
Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–7.
Einar Stefansson TL. Cyclodextrins in eye drop formulations. J Incl Phenom Macrocycl Chem. 2002;44:23–7.
Chang DT, Herceg MC, Bilonick RA, Camejo L, Schuman JS, Noecker RJ. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol. 2009;3:345–55.
Capone Jr A, Singer MA, Dodwell DG, Dreyer RF, Oh KT, Roth DB, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014;34(2):342–51.
Sivaprasad S, McCluskey P, Lightman S. Intravitreal steroids in the management of macular oedema. Acta Ophthalmol Scand. 2006;84(6):722–33.
Umland SP, Schleimer RP, Johnston SL. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm Pharmacol Ther. 2002;15(1):35–50.
Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203(8):1883–9.
Haller JA, Bandello F, Belfort Jr R, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–60.
Elg M, Gustafsson D. A combination of a thrombin inhibitor and dexamethasone prevents the development of experimental disseminated intravascular coagulation in rats. Thromb Res. 2006;117(4):429–37.
Vachon P, Moreau JP. Low doses of dexamethasone decrease brain water content of collagenase-induced cerebral hematoma. Can J Vet Res = Rev Can Rech Vet. 2003;67(2):157–9.
Loftsson T, Hreinsdottir D, Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J Pharm Pharmacol. 2007;59(5):629–35.
Boddu SH, Jwala J, Vaishya R, Earla R, Karla PK, Pal D, et al. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J Ocul Pharmacol Ther: Off J Assoc Ocul Pharmacol Ther. 2010;26(1):37–48.
Ebrahim S, Peyman GA, Lee PJ. Applications of liposomes in ophthalmology. Surv Ophthalmol. 2005;50(2):167–82.
Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18(3):235–9.
Weijtens O, Schoemaker RC, Lentjes EG, Romijn FP, Cohen AF, van Meurs JC. Dexamethasone concentration in the subretinal fluid after a subconjunctival injection, a peribulbar injection, or an oral dose. Ophthalmology. 2000;107(10):1932–8.
Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol. 2008;53(2):139–49.
Yamashita T, Uemura A, Kita H, Sakamoto T. Intraocular pressure after intravitreal injection of triamcinolone acetonide following vitrectomy for macular edema. J Glaucoma. 2007;16(2):220–4.
Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112(1):139–43.
Sheu MT, Chen SY, Chen LC, Ho HO. Influence of micelle solubilization by tocopheryl polyethylene glycol succinate (TPGS) on solubility enhancement and percutaneous penetration of estradiol. J Control Release: Off J Control Release Soc. 2003;88(3):355–68.
Loftsson T, Hreinsdottir D. Determination of aqueous solubility by heating and equilibration: a technical note. AAPS PharmSciTech. 2006;7(1):E4.
Duong AD, Ruan G, Mahajan K, Winter JO, Wyslouzil BE. Scalable, semicontinuous production of micelles encapsulating nanoparticles via electrospray. Langmuir: ACS J Surf Colloids. 2014;30(14):3939–48.
Mitra AK, Velagaleti PR, Natesan S, inventor. Ophthalmic Compositions. USA patent US 13/974,241. 2013.
Craig JP, Simmons PA, Patel S, Tomlinson A. Refractive index and osmolality of human tears. Optom Vis Sci: Off Publ Am Acad Optom. 1995;72(10):718–24.
Janszky I, Vamosi P, Orszagh I, Berta A. Demonstration of increasing standard pH value of lacrimal fluid with increase of flow rate. Acta Ophthalmol Scand. 2001;79(2):180–3.
Dong XY, Meng Y, Feng XD, Sun Y. A metal-chelate affinity reverse micellar system for protein extraction. Biotechnol Prog. 2010;26(1):150–8.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1–2):176–85.
Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713–21.
Hait SK, Moulik SP. Determination of critical micellar concentration of non-ionic surfactants by donor-acceptor interaction with iodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J Surfactant Deterg. 2001;4(3):303–9.
Hariharan S, Minocha M, Mishra GP, Pal D, Krishna R, Mitra AK. Interaction of ocular hypotensive agents (PGF2 alpha analogs-bimatoprost, latanoprost, and travoprost) with MDR efflux pumps on the rabbit cornea. J Ocul Pharmacol Ther: Off J Assoc Ocul Pharmacol Ther. 2009;25(6):487–98.
Acharya G, Lee CH, Lee Y. Optimization of cardiovascular stent against restenosis: factorial design-based statistical analysis of polymer coating conditions. PLoS ONE. 2012;7(8):e43100.
Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388(1–2):107–13.
Zhu H, Chauhan A. Effect of viscosity on tear drainage and ocular residence time. Optom Vis Sci: Off Publ Am Acad Optom. 2008;85(8):715–25.
Cholkar K, Patel A, Dutt Vadlapudi A, Mitra AK. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Patents Nanomed. 2012;2(2):82–95.
Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther: Off J Assoc Ocul Pharmacol Ther. 2013;29(2):106–23.
Mitra AK. In: Mitra AK, editor. Ophthalmic drug delivery systems. New York: Marcel Dekker; 2008.
Gao Y, Li LB, Zhai G. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf B: Biointerfaces. 2008;64(2):194–9.
Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–8.
Lang JC. Ocular drug delivery conventional ocular formulations. Adv Drug Deliv Rev. 1995;16:39–43.
Järvinen KJT, Urtti A. Ocular absorption following topical delivery. Adv Drug Deliv Rev. 1995;16:3–19.
ACKNOWLEDGMENTS
This study has been supported by NIH grants R01EY09171-16, R01EY010659-14 and LUX biosciences, NJ, USA. We would like to thank Dr. Vladimir Dusevich, UMKC School of Dentistry, for helping with the operation of transmission electron microscopy and Mrs. RajyaLaxmi Earla, UMKC school of Pharmacy for helping in NMR sample preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cholkar, K., Hariharan, S., Gunda, S. et al. Optimization of Dexamethasone Mixed Nanomicellar Formulation. AAPS PharmSciTech 15, 1454–1467 (2014). https://doi.org/10.1208/s12249-014-0159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0159-y