Abstract
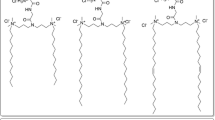
In the present study, nonionic surfactant vesicles (niosomes) formulated with Span 20, cholesterol, and novel synthesized spermine-based cationic lipids with four hydrocarbon tails in a molar ratio of 2.5:2.5:1 were investigated as a gene carrier. The effects of the structure of the cationic lipids, such as differences in the acyl chain length (C14, C16, and C18) of the hydrophobic tails, as well as the weight ratio of niosomes to DNA on transfection efficiency and cell viability were evaluated in a human cervical carcinoma cell line (HeLa cells) using pDNA encoding green fluorescent protein (pEGFP-C2). The niosomes were characterized both in terms of morphology and of size and charge measurement. The formation of complexes between niosomes and DNA was verified with a gel retardation assay. The transfection efficiency of these cationic niosomes was in the following order: spermine-C18 > spermine-C16 > spermine-C14. The highest transfection efficiency was obtained for transfection with spermine-C18 niosomes at a weight ratio of 10. Additionally, no serum effect on transfection efficiency was observed. The results from a cytotoxicity and hemolytic study showed that the cationic niosomes were safe in vitro. In addition, the cationic niosomes showed good physical stability for at least 1 month at 4°C. Therefore, the cationic niosomes offer an excellent prospect as an alternative gene carrier.








Similar content being viewed by others
REFERENCES
Mhashilkar A, Chada S, Roth JA, Ramesh R. Gene therapy: therapeutic approaches and implications. Biotechnol Adv. 2001;19:279–97.
Gabor MR. The future of human gene therapy. Mol Aspects Med. 2001;22:113–42.
Serikawa T, Suzuki N, Kikuchi H, Tanaka K, Kitagawa T. A new cationic liposome for efficient gene delivery with serum into cultured human cells: a quantitative analysis using two independent fluorescent probes. Biochim Biophys Acta. 2000;1467:419–30.
He CX, Tabata Y, Gao JQ. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386:232–42.
Huang Y, Chen J, Chen X, Gao J, Liang W. PEGylated synthetic surfactant vesicles (Niosomes): novel carriers for oligonucleotides. J Mater Sci. 2008;19:607–14.
Manosroi A, Thathang K, Werner RG, Schubert R, Manosroi J. Stability of luciferase plasmid entrapped in cationic bilayer vesicles. Int J Pharm. 2008;356:291–9.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70.
Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmacol Sin B. 2011;1:208–19.
Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interface Sci. 2012;183–184:46–54.
Geusens B, Strobbe T, Bracke S, Dynoodt P, Sanders N, Gele MV, et al. Lipid-mediated gene delivery to the skin. Eur J Pharm Sci. 2011;43:199–211.
Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. J Colloids Surf B: Biointerfaces. 2003;30:129–38.
Vyas SP, Singh RP, Jain S, Mishra V, Mahor S, Singh P, et al. Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int J Pharm. 2005;296:80–6.
Huang Y, Rao Y, Chen J, Yang VC, Liang W. Polysorbate cationic synthetic vesicle for gene delivery. J Biomed Mater Res A. 2011;96:513–9.
Ram IM. Water insoluble and soluble lipids for gene delivery. Adv Drug Deliv Rev. 2005;57:699–712.
Azzama T, Eliyahua H, Makovitzki A, Linial M, Domb AJ. Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. J Control Release. 2004;96:309–23.
Gaucheron J, Santaella C, Vierling P. Transfection with fluorinated lipoplexes based on fluorinated analogues of DOTMA, DMRIE and DPPES. Biochim Biophys Acta. 2002;1564:349–58.
Jean PB, Barbara D, Jean PL, Jose PM. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989;86:6982–6.
Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–96.
Yingyongnarongkul B, Radchatawedchakoon W, Krajarng A, Watanapokasin R, Suksamrarn A. High transfection efficiency and low toxicity cationic lipids with aminoglycerol-diamine conjugate. Bioorg Med Chem. 2009;17:176–88.
Paecharoenchai O, Niyomtham N, Ngawhirunpat T, Rojanarata T, Yingyongnarongkul B, Opanasopit P. Cationic niosomes composed of spermine-based cationic lipids mediate high gene transfection efficiency. J Drug Target. 2012;20:783–92.
Lopes RM, Luísa CM, Eleutério CV, Carvalheiro MC, Scoullica E, Cruz ME. Formulation of ORZ liposomes: in vitro studies and in vivo fate. Eur J Pharm Biopharm. 2012;82:281–90.
Park SI, Lee EO, Yang HM, Park CW, Kim JD. Polymer-hybridized liposomes anchored with alkyl grafted poly(asparagine). J Colloid Interface Sci. 2011;364:31–8.
Weecharangsan W, Opanasopit P, Ngawhirunpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm. 2008;348:161–8.
Paecharoenchai O, Niyomtham N, Apirakaramwong A, Yingyongnarongkul B, Opanasopit P. Effect of acyl chain length of spermine derivatives on transfection efficiency. Adv Mater Res. 2012;506:445–8.
Paecharoenchai O, Niyomtham N, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Yingyongnarongkul B, et al. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech. 2012;13:1302–8.
Huang YZ, Gao JQ, Chen JL, Liang WQ. Cationic liposomes modified with non-ionic surfactants as effective non-viral carrier for gene transfer. Colloids Surf B: Biointerfaces. 2006;49:158–64.
Marzio DL, Marianecci C, Cinque B, Nazzarri M, Cimini AM, Cristiano L, et al. pH-sensitive non-phospholipid vesicle and macrophage-like cells: binding, uptake and endocytotic pathway. Biochim Biophys Acta. 2008;1778:2749–56.
Zhou C, Zhang Y, Yu B, Phelps MA, Lee LJ, Lee RJ. Comparative cellular pharmacokinetics and pharmacodynamics of siRNA delivery by SPANosomes and by cationic liposomes. Nanomedicine. 2013;9(4):504–13.
Liang E, Hughes J. Characterization of a pH-sensitive surfactant, dodecyl-2-(1′-imidazolyl) propionate (DIP), and preliminary studies in liposome mediated gene transfer. Biochim Biophys Acta. 1998;1369:39–50.
Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan: effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22:2075–80.
Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–94.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN) dispersions. Int J Pharm. 1998;168:221–9.
Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24:4283–300.
Paecharoenchai O, Teng L, Yung BC, Teng L, Opanasopit P, Lee RJ. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine. 2013;8(11):1865–73.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Commission of Higher Education (Thailand), the Thailand Research Funds through the Golden Jubilee Ph.D. Program (grant nos. PHD/0092/2551 and PHD/0217/2552), the Office of the Higher Education Commission, Ministry of Education, the Thailand Research Fund (RMU5480003), and the Silpakorn University Research and Development Institute (SURDI 57/01/24) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Paecharoenchai, O., Niyomtham, N., Leksantikul, L. et al. Nonionic Surfactant Vesicles Composed of Novel Spermine-Derivative Cationic Lipids as an Effective Gene Carrier In Vitro . AAPS PharmSciTech 15, 722–730 (2014). https://doi.org/10.1208/s12249-014-0095-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0095-x