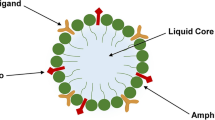
Abstract
Nanoemulsion dosage form serves as a vehicle for the delivery of active pharmaceutical ingredients and has attracted great attention in drug delivery and pharmacotherapy. In particular, nanoemulsions act as an excellent vehicle for poorly aqueous soluble drugs, which are otherwise difficult to formulate in conventional dosage forms. Nanoemulsions are submicron emulsions composed of generally regarded as safe grade excipients. Particle size at the nanoscale and larger surface area lead to some very interesting physical properties that can be exploited to overcome anatomical and physiological barriers associated in drug delivery to the complex diseases such as cancer. Along these lines, nanoemulsions have been engineered with specific attributes such as size, surface charge, prolonged blood circulation, target specific binding ability, and imaging capability. These attributes can be tuned to assist in delivering drug/imaging agents to the specific site of interest, based on active and passive targeting mechanisms. This review focuses on the current state of nanoemulsions in the translational research and its role in targeted cancer therapy. In addition, the production, physico-chemical characterization, and regulatory aspects of nanoemulsion are addressed.





Similar content being viewed by others
REFERENCES
Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2(1):12.
Ganta S, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in parenteral emulsion. Int J Pharm. 2008;360(1–2):115–21.
Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–39.
Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci. 2010;99(11):4630–41.
Ganta S, Sharma P, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J Drug Target. 2010;18(2):125–33.
Dehelean CA, Feflea S, Ganta S, Amiji M. Anti-angiogenic effects of betulinic acid administered in nanoemulsion formulation using chorioallantoic membrane assay. J Biomed Nanotechnol. 2011;7(2):317–24.
Talekar M, Ganta S, Singh A, Amiji M, Kendall J, Denny WA, et al. Phosphatidylinositol 3-kinase inhibitor (PIK75) containing surface functionalized nanoemulsion for enhanced drug delivery, cytotoxicity and pro-apoptotic activity in ovarian cancer cells. Pharm Res. 2012;29(10):2874–86.
Dehelean CA, Feflea S, Gheorgheosu D, Ganta S, Cimpean AM, Muntean D, et al. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J Biomed Nanotechnol. 2013;9(4):577–89.
Ganta S, Devalapally H, Baguley BC, Garg S, Amiji M. Microfluidic preparation of chlorambucil nanoemulsion formulations and evaluation of cytotoxicity and pro-apoptotic activity in tumor cells. J Biomed Nanotechnol. 2008;4(2):165–73.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2007;66(2):227–43.
Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Investig Dermatol. 2007;127(1):154–62.
Patlolla RR, Vobalaboina V. Pharmacokinetics and tissue distribution of etoposide delivered in parenteral emulsion. J Pharm Sci. 2005;94(2):437–45.
Tiwari S, Tan Y-M, Amiji M. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J Biomed Nanotechnol. 2006;2(3–4):3–4.
Gallarate M, Chirio D, Bussano R, Peira E, Battaglia L, Baratta F, et al. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm. 2013;440(2):126–34.
Nesamony J, Shah IS, Kalra A, Jung R. Nebulized oil-in-water nanoemulsion mists for pulmonary delivery: development, physico-chemical characterization and in vitro evaluation. Drug Dev Ind Pharm. 2013. doi:10.3109/03639045.2013.814065.
Hippalgaonkar K, Majumdar S, Kansara V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS PharmSciTech. 2010;11(4):1526–40.
Cockshott ID. Propofol ('Diprivan') pharmacokinetics and metabolism—an overview. Postgrad Med J. 1985;61 Suppl 3:45–50.
Tibell A, Larsson M, Alvestrand A. Dissolving intravenous cyclosporin A in a fat emulsion carrier prevents acute renal side effects in the rat. Transplant Int Off J Eur Soc Organ Transplant. 1993;6(2):69–72.
Gianella A, Jarzyna PA, Mani V, Ramachandran S, Calcagno C, Tang J, et al. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5(6):4422–33.
Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71.
Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–55.
Kim KY. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine Nanotechnol Biol Med. 2007;3(2):103–10.
Ganta S, Deshpande D, Korde A, Amiji M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol Membr Biol. 2010;27(7):260–73.
3Lawler J. Editorial foreword: introduction to the tumour microenvironment review series. J Cell Mol Med. 2009;13(8 A):1403–4.
Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95.
Winter PM, Schmieder, Anne H, Caruthers, Shelton D, Keene, et al. Minute dosages of ανβ3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22(8):2758–67.
Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, et al. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132(12):4259–65.
Devalapally H, Shenoy, Dinesh, Little, Steven, Langer, et al. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;59(4):477–84.
Shenoy D, Little, Steven, Langer, Robert, Amiji, et al. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm Res. 2005;22(12):2107–14.
Na K, Bae YH. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: characterization, aggregation, and adriamycin release in vitro. Pharm Res. 2002;19(5):681–8.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84.
Caron WP, Lay JC, Fong AM, La-Beck NM, Kumar P, Newman SE, et al. Translational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacology. J Pharm Exp Ther. 2013. doi:10.1124/jpet.113.208801.
Gabizon A, Shmeeda, Hilary, Barenholz, Yechezkel. Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42(5):419–36.
Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. Semin Oncol. 2004;31(Supplement 13(0)):5–15.
Wang X, Wang Y-W, Huang Q. Enhancing stability and oral bioavailability of polyphenols using nanoemulsions. Micro/Nanoencapsulation Act Food Ingredients: Am Chem Soc. 2009. p 198–212.
Tagne J-B, Kakumanu S, Ortiz D, Shea T, Nicolosi RJ. A nanoemulsion formulation of tamoxifen increases its efficacy in a breast cancer cell line. Mol Pharm. 2008;5(2):280–6.
Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009;379(2):201–9.
Hoarau D, Delmas P, David S, phanie, Roux E, Leroux J-C. Novel long-circulating lipid nanocapsules. Pharm Res. 2004;21(10):1783–9.
Khalid M, Simard P, Hoarau D, Dragomir A, Leroux J-C. Long circulating poly(ethylene glycol)-decorated lipid nanocapsules deliver docetaxel to solid tumors. Pharm Res. 2006;23(4):752–8.
Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398(1–2):190–203.
Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011;8(1):185–203.
Dimova I, Zaharieva B, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Tissue microarray analysis of EGFR and erbB2 copy number changes in ovarian tumors. Int J Gynecol Cancer. 2006;16(1):145–51.
Lin CK, Chao TK, Yu CP, Yu MH, Jin JS. The expression of six biomarkers in the four most common ovarian cancers: correlation with clinicopathological parameters. APMIS. 2009;117(3):162–75.
Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30(29):5737–50.
Zhang Y, Li J, Lang M, Tang X, Li L, Shen X. Folate-functionalized nanoparticles for controlled 5-fluorouracil delivery. J Colloid Interface Sci. 2011;354(1):202–9.
Zhang C, Zhao LQ, Dong YF, Zhang XY, Lin J, Chen Z. Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur J Pharm Biopharm. 2010;76(1):10–6.
Esmaeili FGM, Ostad S, Atyabi F, Seyedabadi M, Malekshahi M, Amini M, et al. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA–PEG–folate conjugate. J Drug Target. 2008;16(5):415–23.
Zhang HLX, Gao F, Liu L, Zhou Z, Zhang Q. Preparation of folate-modified pullulan acetate nanoparticles for tumor-targeted drug delivery. Drug Deliv. 2010;17(1):48–57.
Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99(14):1095–106.
Kaneda M, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37(10):1922–33.
Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138(3):268–76.
Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48(4):260–70.
Bae P, Jung J, Lim SJ, Kim D, Kim S-K, Chung BH. Bimodal perfluorocarbon nanoemulsions for nasopharyngeal carcinoma targeting. Mol Imaging Biol. 2013;15(4):401–10.
Almeida CP, Vital CG, Contente TC, Maria DA, Maranhao RC. Modification of composition of a nanoemulsion with different cholesteryl ester molecular species: effects on stability, peroxidation, and cell uptake. Int J Nanomedicine. 2010;5:679–86.
Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–65.
Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interface Sci. 2004;107(2–3):125–55.
McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter. 2011;7(6):2297–316.
Donsi F, Annunziata M, Vincensi M, Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159(4):342–50.
Ohguchi Y, Kawano K, Hattori Y, Maitani Y. Selective delivery of folate-PEG-linked, nanoemulsion-loaded aclacinomycin A to KB nasopharyngeal cells and xenograft: effect of chain length and amount of folate-PEG linker. J Drug Target. 2008;16(9):660–7.
Chanamai R, McClements DJ. Impact of weighting agents and sucrose on gravitational separation of beverage emulsions. J Agric Food Chem. 2000;48(11):5561–5.
McClements DJ. Emulsion design to improve the delivery of functional lipophilic components. Annu Rev Food Sci Technol. 2010;1:241–69.
Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release Off J Control Release Soc. 2008;126(3):187–204.
Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204.
Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: a new temperature-sensitive liposome for use with mild hyperthermia. J Liposome Res. 1999;9(4):491–506.
Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13(9):2722–7.
Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. Gold nanoparticles enable selective light-induced contents release from liposomes. J Control Release. 2007;122(1):86–93.
Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–75.
Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–21.
Landon CD, Park JY, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J. 2011;3:38–64.
Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia. 2010;26(5):485–98.
Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. 2006;12(13):4004–10.
Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–7.
Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, et al. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6(9):3748–55.
Wang CY, Huang L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci U S A. 1987;84(22):7851–5.
Connor J, Huang L. pH-sensitive immunoliposomes as an efficient and target-specific carrier for antitumor drugs. Cancer Res. 1986;46(7):3431–5.
Negrini R, Mezzenga R. pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir. 2011;27(9):5296–303.
Mo R, Sun Q, Xue J, Li N, Li W, Zhang C, et al. Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater. 2012;24(27):3659–65.
Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142(2):267–76.
Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–8.
Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(5):626–39.
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–10.
Han J, Washington C. Partition of antimicrobial additives in an intravenous emulsion and their effect on emulsion physical stability. Int J Pharm. 2005;288(2):263–71.
Junghanns J-U, Buttle I, Müller R, Araujo I, Silva A, Egito E, et al. SolEmuls® technology: a way to overcome the drawback of parenteral administration of insoluble drugs. Pharm Dev Technol. 2007;12(5):437–45.
Singh M, Ravin LJ. Parenteral emulsions as drug carrier systems. J Parenter Sci Technol Publ Parenter Drug Assoc. 1986;40(1):34–41.
Hatanaka J, Chikamori H, Sato H, Uchida S, Debari K, Onoue S, et al. Physicochemical and pharmacological characterization of alpha-tocopherol-loaded nano-emulsion system. Int J Pharm. 2010;396(1–2):188–93.
Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103.
Kuntsche J, Horst JC, Bunjes H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int J Pharm. 2011;417(1–2):120–37.
Jiang SP, He SN, Li YL, Feng DL, Lu XY, Du YZ, et al. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J Nanomedicine. 2013;8:3141–50.
Shakeel F, Ramadan W, Ahmed MA. Investigation of true nanoemulsions for transdermal potential of indomethacin: characterization, rheological characteristics, and ex vivo skin permeation studies. J Drug Target. 2009;17(6):435–41.
Shafiq S, Shakeel F. Enhanced stability of ramipril in nanoemulsion containing Cremophor-EL: a technical note. AAPS PharmSciTech. 2008;9(4):1097–101.
Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of Œ≤-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132(3):1221–9.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y, et al. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci. 2008;11(1):167–77.
Modi S, Anderson BD. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol Pharm. 2013;10(8):3076–89.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95.
Fukui H, Koike T, Nakagawa T, Saheki A, Sonoke S, Tomii Y, et al. Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS), with commercial lipid-based formulations. Int J Pharm. 2003;267(1–2):101–12.
Wehrung D, Geldenhuys WJ, Oyewumi MO. Effects of gelucire content on stability, macrophage interaction and blood circulation of nanoparticles engineered from nanoemulsions. Colloids Surf B: Biointerfaces. 2012;94:259–65.
Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–78.
Szebeni J, Alving CR, Rosivall L, Bunger R, Baranyi L, Bedocs P, et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res. 2007;17(2):107–17.
Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol). Langmuir ACS J Surf Colloids. 2006;22(19):8178–85.
Stolnik S, Daudali B, Arien A, Whetstone J, Heald CR, Garnett MC, et al. The effect of surface coverage and conformation of poly(ethylene oxide) (PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. Biochim et Biophys Acta. 2001;1514(2):261–79.
Alvarez-Lorenzo C, Rey-Rico A, Sosnik A, Taboada P, Concheiro A. Poloxamine-based nanomaterials for drug delivery. Frontiers Biosci (Elite edition). 2010;2:424–40.
Maranhao RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197(2):959–66.
Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibañez OCM, et al. Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol. 2005;55(6):565–76.
Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CHM, Rodrigues DG, Maranhão RC. Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol. 2003;55(12):1615–22.
Maranhao RC, Roland IA, Toffoletto O, Ramires JA, Goncalves RP, Mesquita CH, et al. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids. 1997;32(6):627–33.
Pinheiro KV, Hungria VT, Ficker ES, Valduga CJ, Mesquita CH, Maranhao RC. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin's and non-Hodgkin's lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother Pharmacol. 2006;57(5):624–30.
Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, et al. Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol. 2002;49(6):487–98.
Béduneau A, Saulnier P, Hindré F, Clavreul A, Leroux J-C, Benoit J-P. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’ fragments. Biomaterials. 2007;28(33):4978–90.
Remmer H. The role of the liver in drug metabolism. Am J Med. 1970;49(5):617–29.
Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (London, England). 2008;3(5):703–17.
Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70.
FDA. Q8(R1) Pharmaceutical development revision 1. International Conference on Harmonisation (ICH) Q8 Guideline 2008.
FDA. Guidance for Industry Q9 Quality Risk management. International Conference on Harmonisation (ICH) Q9 Guideline. 2006. http://www.fda.gov/.
FDA. Guidance for Industry Q10 Pharmaceutical Quality System. International Conference on Harmonisation (ICH) Q10 Guideline 2009. http://www.fda.gov/.
ACKNOWLEDGEMENTS
The authors are thankful to the support by the National Cancer Institute of the National Institutes of Health grant U54-CA151881.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Mahavir B. Chougule and Chalet Tan
Rights and permissions
About this article
Cite this article
Ganta, S., Talekar, M., Singh, A. et al. Nanoemulsions in Translational Research—Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 15, 694–708 (2014). https://doi.org/10.1208/s12249-014-0088-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0088-9