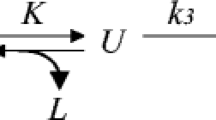Abstract
Sodium caprylate was added to a pharmaceutical-grade human serum albumin (HSA) to stabilize the product. In this study we have aimed to establish how caprylate ligand protects HSA from thermal degradation. The fatty acid stabilizer was first removed from commercial HSA by charcoal treatment. Cleaned HSA was made to 10% w/v in pH 7.4 buffered solutions and doped with sodium caprylate in serial concentrations up to 0.16 mmol/g-protein. These solutions as well as a commercial HSA, human serum, and enriched-albumin fraction were subjected to differential scanning calorimetry (DSC) within the temperature range of 37–90°C at a 5.0°C/min scanning rate. The globular size of the cleaned HSA solutions was measured by dynamic light scattering. The denaturing temperatures for albumin with sodium caprylate and a commercial one were significantly higher than for albumin only. It was found that the protein globules of cleaned HSA were not as stable as that of the native one due to aggregation, and the caprylate ion may reduce the aggregation by enlarging the globules’ electrical double layer. A rational approximation of the Lumry-Eyring protein denaturation model was used to treat DSC denaturing endotherms. The system turned from irreversible dominant Scheme: \( N\overset{{\displaystyle {k}_3}K}{\to }P \) to reversible dominant Scheme: \( N\overset{{\displaystyle {k}_1}}{\to }P \) with the increase in caprylate concentration from null to ~0.08 mmol/g-protein. It was postulated that the caprylate ligand may decrease the rate of reversible unfolding as it binds to the IIIA domain which is prone to reversible unfolding/refolding and causes further difficulty for irreversible denaturation which, in turn, HSA can be stabilized.








Similar content being viewed by others
REFERENCES
Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–90.
Olsen H, Andersen A, Kongsgaard UE, Bormer OP. Pharmaceutical-grade albumin: impaired drug-binding capacity in vitro. BMC Clin Pharmacol. 2004. doi:10.1186/1472-6904-4-4.
Albumin (Human) USP 20% solution: Albutein® prescribing information, Grifols Barcelona, Spain, 2008.
Anraku M, Tsurusaki Y, Watanabe H, Maruyama T, Kragh-Hansen U, Otagiri M. Stabilizing mechanisms in commercial albumin preparations: octanoate and N-acetyl-l-tryptophanate protect human serum albumin against heat and oxidative stress. Biochim Biophys Acta. 2004;1702:9–17.
Arakawa T, Kita Y. Stabilizing effects of caprylate and acetyltryptophanate on heat-induced aggregation of bovine serum albumin. Biochim Biophys Acta. 2000;1479:32–6.
Lumry R, Eyring H. Conformation changes of proteins. J Phys Chem. 1954;58:110–20.
Sanchez-Ruiz JM. Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry. Biophys J. 1992;61:921–35.
Pico GA. Thermodynamic feature of the thermal unfolding of human serum albumin. Int J Biol Macromol. 1997;20:63–73.
Khrapunov S, Brenowitz M. Stability, denaturation and refolding of Mycobacterium tuberculosis MfpA, a DNA mimicking protein that confers antibody resistant. Biophys Chem. 2011;159:33–40.
Gundry RL, Fu Q, Jelinek CA, Van Eyk JE, Cotter RJ. Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteomics Clin Appl. 2007;1:73–88.
Chen RF. Removal of fatty acid from serum albumin by charcoal treatment. J Biol Chem. 1967;242:173–81.
Dengler T, Kellner S, Furst G. Quantitative determination of sodium-octanoate in human serum albumin preparations. Infusionstherapie. 1988;15:273–4.
Flora K, Brennan JD, Baker GA, Doody MA, Bright FV. Unfolding of acrylodan-labeled human serum albumin probed by steady- state and time-resolved fluorescence methods. Biophys J. 1998;75:1084–96.
Garbett NC, Mekmaysy CS, Helm CW, Jenson AB, Chaires JB. Differential scanning calorimetry of blood plasma for clinical diagnosis and monitoring. Exp Mol Pathol. 2009;86:186–91.
Shrake A, Ross PD. Biphasic denaturation of human albumin due to ligand redistribution during unfolding. J Biol Chem. 1988;263:15392–9.
Privalov PL. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104.
Mitra RK, Sinha SS, Pal SK. Hydration in protein folding: thermal unfolding/refolding of human serum albumin. Langmuir. 2007;23:10224–9.
Jachimska B, Wasilewska M, Adamczyk Z. Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir. 2008;24:6866–72.
Kragh-Hansen U. Octanoate binding to the indole- and benzodiazepine-binding region of human seruim albumin. Biochem J. 1991;273:641–4.
Varshney A, Sen P, Ahmad E, Rehan M, Subbarao N, Khan RH. Ligand binding strategies of human serum albumin: how can the cargo be utilized? Chirality. 2010;22:77–87.
Lee IY, McMenamy RH. Location of the medium chain fatty acid site on human serum albumin. Residues involved and relationship to the indole site. J Biol Chem. 1980;255:6121–7.
Celej MS, Dassie SA, Gonzalez M, Bianconi ML, Fidelio GD. Differential scanning calorimetry as a tool to estimate binding parameters in multiligand binding proteins. Anal Biochem. 2006;350:277–84.
Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 1999;12:439–46.
Cordes AA, Platt CW, Carpenter JF, Randolph TW. Selective domain stabilization as a strategy to reduce fusion protein aggregation. J Pharm Sci. 2012;101:1400–9.
ACKNOWLEDGMENTS
The authors are grateful to the Royal Golden Jubilee Ph. D. program for partially financial support and Prince of Songkla University (PSU) for the research grant. Special thanks go to PSU Scientific Equipment Center, and Drug Delivery System Excellent Center, Faculty of Pharmaceutical Sciences for Lab facilities. And, the authors would like to thank Dr. Brian Hodgson for English language revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faroongsarng, D., Kongprasertkit, J. The Role of Caprylate Ligand Ion on the Stabilization of Human Serum Albumin. AAPS PharmSciTech 15, 465–471 (2014). https://doi.org/10.1208/s12249-014-0076-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0076-0