Abstract
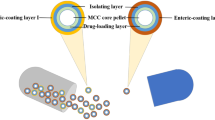
The aim of the study was to develop single-unit tablet in capsule system of aceclofenac for the treatment of late night pain and morning stiffness associated with rheumatoid arthritis. The system was conceptualized as a three-component design (1) a hard gelatin enteric-coated capsule (for carrying two pulses), (2) first-pulse granules (for rapid release in intestine), and (2) second-pulse matrix tablet (for slow release in colon). An appropriate integration of pH-sensitive (Eudragit S100) and bacteria-responsive (inulin) functions, on the basis of 32 factorial design, led to formulation of TICS 1–9 that were screened for in vitro release. TICS 2 with biphasic drug release of 98.64% from first-pulse granules in simulated intestinal fluid (12 h) and 97.82% from second-pulse matrix tablet in simulated colonic fluid (24 h) was the optimized formulation that exhibited Fickian diffusion of drug (n = 0.363). In vivo fluoroscopy in rats traced the intact tablet to colon in 7.5 h that got eroded at the tenth hour. This demonstrated the colon-specific delivery of the matrix tablet affirming the potential of the system to obviate the need for two-time administration of drug at odd hours. The experimental design was validated by extra design check point, and diffuse reflectance spectroscopy and DSC revealed absence of chemical interaction between the formulation excipients.






Similar content being viewed by others
REFERENCES
Zhu Z, Zheng L. Development and mathematical simulation of theophylline pulsatile release tablets. Drug Dev Ind Pharm. 2005;31:1009–17.
Smolenska Z, Kaznowska Z, Zarowny D, Simmonds HA, Smolenski RT. Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology (Oxford). 1999;38:997–1002.
Sharma P, Pathak K. Are biological targets the final goal for rheumatoid arthritis therapy? Expert Opin Biol Targets. 2012;12(12):1611–22.
Saraf S. http://www.pharmainfo.net/reviews/aceclofenac-potent-non-steroidal-anti-inflammatory-drug (2012). Accessed on 18th Dec 2011.
Neagu C, Bahrim G. Inulinases—versatile tool for biotechnology. Innov Rom Food Biotechnol. 2011;9:1–11.
Akhgari A, Afrasiabi GH, Sadeghi F. Combination of inulin and time dependent polymethacrylates as a coating system to achieve colonic delivery of indomethacin. DARU J Pharm Sci. 2009;17:199–209.
Barclay T, Milena GM, Cooper P, Petrovsky N. Inulin—versatile polysaccharide with multiple pharmaceutical and food chemical uses. J Excipients Food Chem. 2010;1(3):27–55.
Aulton ME. Powder flow in pharmaceutics—the science of dosage form design. London: Churchill Livingstone; 2002. p. 114–6.
Ministry of Health and Family Welfare. Indian pharmacopoeia. Ghaziabad: The Indian Pharmacopoeial Commission; 2007. p. 1648–50.
United States Pharmacopoeia 27/National Formulary 24. Rockville, MD: US Pharmacopoeia Convention, Inc; 2000. pp. 2524–5.
Gupta VK, Beckert TE, Deusch NJ, Harinaraon M, Price JC. A novel pH and time based multi potential colonic drug delivery system. Int J Pharm. 2001;213:83–91.
Gao C, Huang J, Jiao Y, Shan L, Liu Y, Li Y, et al. In vitro release and in vivo absorption in beagle dogs of meloxicam from Eudragit FS30D coated pellets. Int J Pharm. 2006;322:104–12.
Kshirsagar SJ, Bhalekar MR, Umap RR. In vitro in vivo comparison of two pH sensitive eudragit polymers for colon specific drug delivery. J Pharm Sci Res. 2009;1:61–70.
Bussemer T, Peppas NA, Bodmeier R. Time-dependent mechanical properties of polymeric coatings used in rupturable pulsatile release dosage forms. Drug Dev Ind Pharm. 2003;29:623–30.
Shivkumar HN, Sarasiji S, Desai BG. Design and evaluation of pH sensitive multiparticulate system for choronotherapeutic delivery of diltiazem hydrochloride. Ind J Pharm Sci. 2006;68(6):781–7.
Mohamad A, Dashevsky A. In vitro and in vivo performance of a multiparticulate pulsatile drug delivery system. Drug Dev Ind Pharm. 2007;33:113–9.
Koteshwara KB, Thoppil SC, Naha A. Design and development of multiparticulate drug delivery of metronidazole for targeted delivery to colon. Int J Pharm. 2011;3:3580–9.
Shah N, Patel M, Shah T, Amin A. Design, development and optimization of colon targeted drug delivery system for Crohn’s disease. J Pharm Educ Res. 2011;2:42–50.
Meghal AK, Chaudari PS, Mathur VB. Formulation and evaluation of enteric coated HPMC capsule of diclofenac sodium. Res J Pharm Biol Chem Sci. 2011;2:790–8.
Kamel S, Ali N, Jahangir K, Shah SM, El-Gendy AA. Pharmaceutical significance of cellulose: a review. Express Polym Lett. 2008;2:758–78.
Tukaram BN, Rajagopalan IV, Sharatchandra PSI. The effects of lactose, microcrystalline cellulose and dicalcium phosphate on swelling and erosion ofcompressed HPMC matrix tablets: texture analyzer. Iran J Pharm Res. 2010;9(4):349–58.
Chambin O, Champion D, Debray C, Rochat-Gonthier MH, Le MM, Pourcelot Y. Effects of different cellulose derivatives on drug release mechanism at a preformulation stage. J Control Release. 2004;95:101–8.
Coben LJ, Lieberman HA, Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Mumbai: Varghese; 1991. p. 241–50.
Mohsen A, Khoweysa OM, Shoukri RA. Optimization of aceclofenac once daily matrix tablets: in vitro and in vivo studies. J Pharm Res Opin. 2011;2:12–22.
Ciolacu D, Ciolacu F, Popa VI. Amorphous cellulose—structure and characterization. Cellul Chem Technol. 2011;45(1–2):13–21.
Azarmi S, Farid J, Nokhodchi A, Bahari-Saravi SM, Valizadeh H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices. Int J Pharm. 2002;246:171–7.
Eissens AC, Bolhuis GK, Hinrichs WLJ, Frijlink HW. Inulin as filler—binder for tablets prepared by direct compaction. Eur J Pharm Sci. 2002;15:31–8.
Srivastava R, Kumar D, Pathak K. Colonic luminal surface retention of meloxicam microsponges delivered by erosion based colon targeted matrix tablet. Int J Pharm. 2012;427:153–62.
Vats A, Pathak K. Tabletted guar gum microspheres of piroxicam for targeted adjuvant therapy for colonic adenocarcinomas. Ther Deliv. 2012;3(11):1281–95.
Roberfroid M. Introducing inulin type fructans. Br J Nutr. 2005;93 Suppl 1:S13–25.
Yasuda K, Maiorano R, Welch RM, Miller DD, Lei XG. Ceacum is major degradation site of ingested inulin in young pigs. J Nutr. 2007;137(11):2399–404.
Liberman HA, Lachman L, Schwartz JB. Pharmaceutical dosage forms; tablets (2). New York: Mercel Dekker; 1989. p. 210.
Kumar R, Patil SR, Patil MB, Paschapur MS, Mahalaxami R. Design and characterization of aceclofenac mouth dissolving tablets by effervescent formulation approach. Der Pharmacia Lettre. 2010;2:220–36.
Qureshi J, Ahuja A, Baboota S, Chutani K, Jain S, Ali J. Development and evaluation of a time-specific pulsatile-release tablet of aceclofenac: a solution for morning pain in rheumatoid arthritis. Methods Find Exp Clin Pharmacol. 2009;31(1):15–23.
ACKNOWLEDGMENTS
The authors are thankful to Ranbaxy Research Laboratory, Gurgaon, India for providing aceclofenac as gift sample and to All India Council for Technical Education for providing final assistance to carry out research work. The authors are highly indebted to Prof Satish Kumar Garg, Dean, Pt. Deen Dayal Upadhayaya Veterinary University, Mathura, India for extending the facilities for in vivo roentgenography study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Pathak, K. Inulin-Based Tablet in Capsule Device for Variable Multipulse Delivery of Aceclofenac: Optimization and In Vivo Roentgenography. AAPS PharmSciTech 14, 736–747 (2013). https://doi.org/10.1208/s12249-013-9959-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-9959-8