Abstract
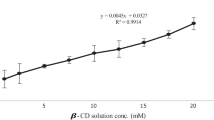
SN-38, an active metabolite of irinotecan, is up to 1,000-fold more potent than irinotecan. But the clinical use of SN-38 is limited by its extreme hydrophobicity and instability at physiological pH. To enhance solubility and stability, SN-38 was complexed with different cyclodextrins (CDs), namely, sodium sulfobutylether β-cyclodextrin (SBEβCD), hydroxypropyl β-cyclodextrin, randomly methylated β-cyclodextrin, and methyl β-cyclodextrin, and their influence on SN-38 solubility, stability, and in vitro cytotoxicity was studied against ovarian cancer cell lines (A2780 and 2008). Phase solubility studies were conducted to understand the pattern of SN-38 solubilization. SN-38-βCD complexes were characterized by differential scanning calorimetry (DSC), X-ray powder diffraction analysis (XRPD), and Fourier transform infrared (FTIR). Stability of SN-38-SBEβCD complex in pH 7.4 phosphate-buffered saline was evaluated and compared against free SN-38. Phase solubility studies revealed that SN-38 solubility increased linearly as a function of CD concentration and the linearity was characteristic of an AP-type system. Aqueous solubility of SN-38 was enhanced by about 30–1,400 times by CD complexation. DSC, XRPD, and FTIR studies confirmed the formation of inclusion complexes, and stability studies revealed that cyclodextrin complexation significantly increased the hydrolytic stability of SN-38 at physiological pH 7.4. Cytotoxicity of SN-38-SBEβCD complex was significantly higher than SN-38 and irinotecan in both A2780 and 2008 cell lines. Results suggest that SBEβCD encapsulated SN-38 deep into the cavity forming stable inclusion complex and as a result increased the solubility, stability, and cytotoxicity of SN-38. It may be concluded that preparation of inclusion complexes with SBEβCD is a suitable approach to overcome the solubility and stability problems of SN-38 for future clinical applications.









Similar content being viewed by others
References
Meyer-Losic F, Nicolazzi C, Quinonero J, Ribes F, Michel M, Dubois V, et al. DTS-108, a novel peptidic prodrug of SN38: in vivo efficacy and toxicokinetic studies. Clin Cancer Res. 2008;14(7):2145–53. PubMed PMID: 18381956. Epub 2008/04/03. eng.
Venkata K Yellepeddi, Kiran K Vangara, Srinath Palakurthi. In-vivo efficacy of PAMAM dendrimers-cisplatin complexes in SKOV-3 xenografted Balb/c nude mice. J Biotechnol Biomater. 2013, 3(2): S13:003.
Zhao H, Rubio B, Sapra P, Wu D, Reddy P, Sai P, et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19(4):849–59. PubMed PMID: 18370417. Epub 2008/03/29. eng.
Ebrahimnejad P, Dinarvand R, Sajadi A, Jaafari MR, Nomani AR, Azizi E, et al. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomedicine. 2010;6(3):478–85. PubMed PMID: 19836467. Epub 2009/10/20. eng.
Kiran KV, Jingbo LL, Srinath P. Hyaluronic Acid-decorated PLGA-PEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian. Anticancer Res. 2013, 33(6): 2425–34.
Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, et al. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270(1–2):93–107. PubMed PMID: 14726126. Epub 2004/01/17. eng.
Gu Q, Xing JZ, Huang M, He C, Chen J. SN-38 loaded polymeric micelles to enhance cancer therapy. Nanotechnology. 2012;23(20):205101. PubMed PMID: 22543761. Epub 2012/05/01. eng.
Takahashi A, Ohkohchi N, Yasunaga M, Kuroda J, Koga Y, Kenmotsu H, et al. Detailed distribution of NK012, an SN-38-incorporating micelle, in the liver and its potent antitumor effects in mice bearing liver metastases. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(19):4822–31. PubMed PMID: 20807756.
Carie A, Rios-Doria J, Costich T, Burke B, Slama R, Skaff H, et al. IT-141, a polymer micelle encapsulating SN-38. Induces tumor regression in multiple colorectal cancer models. J Drug Deliv. 2011;2011:869027. PubMed PMID: 22187652. Pubmed Central PMCID: 3236496.
Patnaik A, Papadopoulos KP, Tolcher AW, Beeram M, Urien S, Schaaf LJ, et al. Phase I dose-escalation study of EZN-2208 (PEG-SN38), a novel conjugate of poly(ethylene) glycol and SN38, administered weekly in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;71(6):1499–506. PubMed PMID: 23543270.
Vijayalakshmi N, Ray A, Malugin A, Ghandehari H. Carboxyl-terminated PAMAM-SN38 conjugates: synthesis, characterization, and in vitro evaluation. Bioconjug Chem. 2010;21(10):1804–10. PubMed PMID: 20836544. Pubmed Central PMCID: 3197241. Epub 2010/09/15. eng.
Serafino A, Zonfrillo M, Andreola F, Psaila R, Mercuri L, Moroni N, et al. CD44-targeting for antitumor drug delivery: a new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr Cancer Drug Targets. 2011;11(5):572–85. PubMed PMID: 21486216. Epub 2011/04/14. eng.
Loftsson T, Jarho P, Masson M, Jarvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–51. PubMed PMID: 16296758. Epub 2005/11/22. eng.
Battu SK, Repka MA, Maddineni S, Chittiboyina AG, Avery MA, Majumdar S. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech. 2010;11(3):1466–75. PubMed PMID: 20842541. Pubmed Central PMCID: 2974104. Epub 2010/09/16. eng.
Sathigari S, Chadha G, Lee YH, Wright N, Parsons DL, Rangari VK, et al. Physicochemical characterization of efavirenz-cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009;10(1):81–7. PubMed PMID: 19148759. Pubmed Central PMCID: 2663670. Epub 2009/01/17. eng.
Saetern AM, Nguyen NB, Bauer-Brandl A, Brandl M. Effect of hydroxypropyl-beta-cyclodextrin-complexation and pH on solubility of camptothecin. Int J Pharm. 2004;284(1–2):61–8. PubMed PMID: 15454297. Epub 2004/09/30. eng.
Kang J, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur J Pharm Sci. 2002;15(2):163–70. PubMed PMID: 11849913. Epub 2002/02/19. eng.
Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, et al. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012;29(7):1775–86. PubMed PMID: 22322899. Epub 2012/02/11. eng.
Grosse PY, Bressolle F, Pinguet F. In vitro modulation of doxorubicin and docetaxel antitumoral activity by methyl-beta-cyclodextrin. Eur J Cancer. 1998;34(1):168–74. PubMed PMID: 9624253. Epub 1998/06/13. eng.
Bouquet W, Boterberg T, Ceelen W, Pattyn P, Peeters M, Bracke M, et al. In vitro cytotoxicity of paclitaxel/beta-cyclodextrin complexes for HIPEC. Int J Pharm. 2009;367(1–2):148–54. PubMed PMID: 18938234, Epub 2008/10/22. eng.
Manaspon C, Nittayacharn P, Vejjasilpa K, Fongsuk C, Nasongkla N. SN-38:beta-cyclodextrin inclusion complex for in situ solidifying injectable polymer implants. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3241–4. PubMed PMID: 22255030. Epub 2012/01/19. eng.
Xuan T, Zhang JA, Ahmad I. HPLC method for determination of SN-38 content and SN-38 entrapment efficiency in a novel liposome-based formulation, LE-SN38. J Pharm Biomed Anal. 2006;41(2):582–8. PubMed PMID: 16386867. Epub 2006/01/03. eng.
Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC. Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol. 2002;55(5):294–9. PubMed PMID: 12354931. Pubmed Central PMCID: 1187258.
Loftsson T, Brewster ME. Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci. 2012;101(9):3019–32. PubMed PMID: 22334484. Epub 2012/02/16. eng.
Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302(1–2):18–28. PubMed PMID: 16099118. Epub 2005/08/16. eng.
Kang J, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur J Pharm Sci Off J Eur Fed Pharma Sci. 2002;15(2):163–70. PubMed PMID: 11849913.
Yellepeddi VK, Vangara KK, Kumar A, Palakurthi S. Comparative evaluation of small-molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cells. Anticancer Res. 2012;32(9):3651–8. PubMed PMID: 22993302. Epub 2012/09/21. eng.
Kumar A, Yellepeddi VK, Vangara KK, Strychar KB, Palakurthi S. Mechanism of gene transfection by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. J Drug Target. 2011;19(9):770–80. PubMed PMID: 21457075. Epub 2011/04/05. eng.
Jwala J, Boddu SH, Shah S, Sirimulla S, Pal D, Mitra AK. Ocular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovir. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2011;27(2):163–72. PubMed PMID: 21500985. Pubmed Central PMCID: 3078652.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62(11):1607–21. PubMed PMID: 21039545. Epub 2010/11/03. eng.
Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69(13):5292–3. PubMed PMID: 19549886.
Hillman EM, Moore A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nat Photonics. 2007;1(9):526–30. PubMed PMID: 18974848. Pubmed Central PMCID: 2575379.
Jain AS, Date AA, Pissurlenkar RR, Coutinho EC, Nagarsenker MS. Sulfobutyl ether(7) beta-cyclodextrin (SBE(7) beta-CD) carbamazepine complex: preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS PharmSciTech. 2011;12(4):1163–75. PubMed PMID: 21918921. Pubmed Central PMCID: 3225538.
Coates J (2000) Interpretation of infrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry, Wiley, New York
Cirri M, Maestrelli F, Corti G, Furlanetto S, Mura P. Simultaneous effect of cyclodextrin complexation, pH, and hydrophilic polymers on naproxen solubilization. J Pharm Biomed Anal. 2006;42(1):126–31. PubMed PMID: 16406448. Epub 2006/01/13. eng.
Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Blair A. Use of hair coloring products and the risk of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. Am J Public Health. 1992;82(7):990–7. PubMed PMID: 1609918. Pubmed Central PMCID: 1694062. Epub 1992/07/01. eng.
Maroju RK, Turner DC, Zhang H. Solubilizing efficiency and in vitro cytotoxicity of peptoad G. J Pharm Sci. 2010;99(4):2196–8. PubMed PMID: 19739113.
Acknowledgments
This work was partially supported by Texas A&M Health Science Center Cancer Research Council Seed Grant to SP.
Conflict of Interest
Authors declare no conflict of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vangara, K.K., Ali, H.I., Lu, D. et al. SN-38-Cyclodextrin Complexation and Its Influence on the Solubility, Stability, and In Vitro Anticancer Activity Against Ovarian Cancer. AAPS PharmSciTech 15, 472–482 (2014). https://doi.org/10.1208/s12249-013-0068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0068-5