Abstract
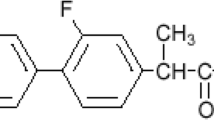
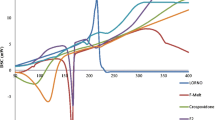
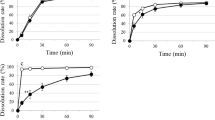
The interest in and need for formulating miconazole nitrate (MN), a broad-spectrum antifungal, as an oral disintegrating tablet for treatment of some forms of candidiasis have increased. Formulation of MN in this dosage form will be more advantageous, producing dual effect: local in the buccal cavity and systemic with rapid absorption. Four formulations were prepared utilizing the foam granulation technique. The prepared tablets were characterized by measuring the weight uniformity, thickness, tensile strength, friability, and drug content. In addition, tablet disintegration time, in vitro dissolution, and in vivo disintegration time were also evaluated. Stability testing for the prepared tablets under stress and accelerated conditions in two different packs were investigated. Each pack was incubated at two different elevated temperature and relative humidity (RH), namely 40 ± 2°C/75 ± 5% RH and 50 ± 2°C/75 ± 5% RH. The purpose of the study is to monitor any degradation reactions which will help to predict the shelf life of the product under the defined storage conditions. Finally, in vivo study was performed on the most stable formula to determine its pharmacokinetic parameters. The results revealed that all the prepared tablets showed acceptable tablet characteristics and were stable under the tested conditions. The most stable formula was that containing magnesium stearate as lubricant, hydrophobic Aerosil R972 as glidant, low urea content, mannitol/microcrystalline cellulose ratio 2:1, and 9% Plasdone XL100 as superdisintegrant. The in vivo results revealed that the tested formula showed rapid absorption compared to the physical blend (t max were 1 and 4 h, respectively), while the extent of absorption was almost the same.











Similar content being viewed by others
REFERENCES
Himanshu KS, Tarashankar B, Jalaram HT, Chirag AP. Recent advances in granulation technology. Int J Pharm Sci Rev Res. 2010;5(3):Article-008.
Sandler N, Lammens RF. Pneumatic dry granulation: potential to improve roller compaction technology in drug manufacture. Expert Opin Drug Deliv. 2011;8(2):225–36.
Powder pro (2012) http://www.powderpro.se/products/technology/. Accessed 8 Apr 2012.
Ochoa L, Igartua M, Hernández RM, Gascón AR, Pedraz JL. Preparation of sustained release hydrophilic matrices by melt granulation in a high-shear mixer. J Pharm Pharm Sci. 2005;8(2):132–40.
Rodriguez L, Cavallari C, Passerini N, Albertini B, González-Rodríguez M, Fini A. Preparation and characterization by morphological analysis of diclofenac/PEG 4000 granules obtained using three different techniques. Int J Pharm. 2002;242(1–2):285–9.
Ismat U, Jennifer W, Shih-Ying C, Gary JW, Nemichand BJ, San K. Moisture activated dry granulation—part I: a guide to excipient and equipment selection and formulation development. Pharm Technol. 2009;33(11):62–70.
Lin HL, Ho HO, Chen CC, Yeh TS, Sheu MT. Process and formulation characterizations of the thermal adhesion granulation (TAG) process for improving granular properties. Int J Pharm. 2008;357(1–2):206–12.
Paul J, Shesky R, Colin K. New foam binder technology from Dow improves granulation process. Pharm Can. 2006; 19–22.
Bouckaert S, Remon JP. In vitro bioadhesion of a buccal, miconazole slow release tablets. J Pharm Pharmacol. 1993;45:504–7.
Minghetti P, Cilurzo F, Casiraghi A, Molla FA, Montanari L. Dermal patches for the controlled release of miconazole: influence of the drug concentration on the technological characteristics. Drug Dev Ind Pharm. 1999;25:679–84.
Mandal TK. Swelling-controlled release system for the vaginal delivery of miconazole. Eur J Pharm Biopharm. 2000;50:337–43.
Pedersen M, Rassing MR. Miconazole chewing gum as a drug delivery system: test of release promoting additives. Drug Dev Ind Pharm. 1991;17:411–20.
Bouckaert S, Schautteet H, Lefebvre RA, Remon JP, van Clooster R. Comparison of salivary miconazole concentrations after administration of a bioadhesive slow-release buccal tablet and an oral gel. Eur J Clin Phamacol. 1992;43:137–40.
ICH. Stability testing of new drug substances and products. Geneva: International Conference on Harmonization, IFPMA; 1993.
ICH. Impurities in new drug products. Geneva: International Conference on Harmonization, IFPMA; 1996.
Hui Y, Huang NH, Ebbert L, Bina H, Chiang A, Maples C, Pritt M, Kern T, Patel N. Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. J Pharmacol Toxicol Methods. 2007;56:256–64.
EMEA, The European Agency for the Evaluation of Medicinal Products, Human Medicines Evaluation Unit. Committee for Proprietary Medicinal Products (CPMP). Note for guidance on quality of modified release products. A: oral dosage forms. B: transdermal dosage forms, Section I (quality). 1999. CPMP/QWP/604/96, 1–15.
USP 28, the United States Pharmacopoeia, 28th Revision. Rockville, MD: Pharmacopoeial Convention, Inc. 2005.
Burgess DJ, Crommelin DJA, Hussain AS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: EUFEPS workshop report. AAPS PharmSci. 2004;6(1):Article 11.
Harshal AP, Priscilla MD. Development and evaluation of herbal laxative granules. J Chem Pharm Res. 2011;3(3):646–50.
Aulton ME. Pharmaceutics: the science of dosage form design. 2nd ed. New York: Churchill Livingstone; 2002. p. 154–5.
Wells J. Pharmaceutical preformulation. In: Aulton ME, editor. Pharmaceutics the science of dosage form design. 2nd ed. Edinburgh: Churchill Livingstone; 2003. p. 133–4.
Haririan I, Newton JM. Tensile strength of circular flat and convex-faced avicel PH102 tablets. Daru. 1999;7(3):36–9.
USFDA. Dissolution methods database. 2010. accessdata.fda.gov/scripts/cder/dissolution. Accessed on 15 Jun 2010.
Okuda Y, Irisawa Y, Okimoto K, Osawa T, Yamashita S. A new formulation for orally disintegrating tablets using a suspension spray-coating method. Int J Pharm. 2009;382:80–7.
USFDA. Bioequivalence guidelines. 2010. accessdata.fda.gov/scripts/cder/dissolution. Accessed 15 Jun 2010.
Kobylifiska M, Kobylifiska K, Sobik B. High-performance liquid chromatographic analysis for the determination of miconazole in human plasma using solid phase extraction. J Chromatogr B Biomed Appl. 1996;685:191–5.
Sheskey P, Keary C, Clark D, Balwinski K. Scale up formulae of foam granulation technology-high shear. Pharm Technol. 2007;31(4):94–9.
Barillaro V, Bertholet P, Sandrine HH. Effect of acidic ternary compounds on the formation of miconazole/cyclodextrin inclusion complexes by means of supercritical carbon dioxide. J Pharm Pharm Sci. 2004;7(3):378–88.
Barillaro V, Dive G, Bertholet P, Evrard B, Delattre L, Frederich M, Ziemons E, Piel G. Theoretical and experimental investigations of organic acids/ cyclodextrin complexes and their consequences upon the formation of miconazole/previous termcyclodextrin/acid ternary inclusion complexes. Int J Pharm. 2007;342:152–60.
Yoshinari T, Forbes RT, York P, Kawashima Y. The improved compaction properties of mannitol after a moisture-induced polymorphic transition. Int J Pharm. 2003;258:121–31.
Nie X. Heat and moisture migration within a porous urea particle bed. PhD thesis, University of Saskatchewan, 2010; p. 8–9.
USP, the United States Pharmacopoeia. 34th edn., Rockville, MD: Pharmacopoeial Convention, Inc. 2011.
Lerk CF, Lagas M, Fell JT, Nauta P. Effect of hydrophilization of hydrophobic drugs on release rate from capsules. J Pharm Sci. 1978;67(7):935–9.
Jafari MR, Danti AG, Ahmed I. Comparison of polyethylene glycol, polyvinylpyrrolidone and urea as excipients for solid dispersion systems of MN. Int J Pharm. 1988;48:207–15.
ICH. International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use, by the ICH Steering Committee, version 2, 2005.
The European Pharmacopoeia (Ph.Eur.). 6th ed., Strasbourg, France: European Directorate for the Quality of Medicines, Council of Europe. 2010.
Faroongsarng D, Peck GE. Thermal porosity analysis of croscarmellose sodium and sodium starch glycolate by differential scanning calorimetry. AAPS Pharma Sci Tech. 2003;4:E67.
Stubberud L, Arwidsson HG, Graffner C. Water–solid interactions: a technique for studying moisture sorption/ desorption. Int J Pharm. 1995;114:55–64.
Rowe RC, Shesky PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press; 2009. p. 196–8.
Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317:61–8.
Barillaro V, Bertholet P, Sandrine HH. Effect of acidic ternary compounds on the formation of miconazole/cyclodextrin inclusion complexes by means of supercritical carbon dioxide. J Pharm Sci. 2004;7(3):378–88.
ACKNOWLEDGMENTS
The authors highly appreciated the cooperation of DEEF Pharmaceutical Industries Company for giving the research team the permission to use the foam granulation machine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, T.A., El-Say, K.M., Mahmoud, M.F. et al. Miconazole Nitrate Oral Disintegrating Tablets: In Vivo Performance and Stability Study. AAPS PharmSciTech 13, 760–771 (2012). https://doi.org/10.1208/s12249-012-9798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9798-z