Abstract
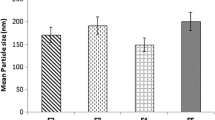
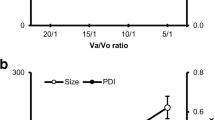
Solid lipid nanoparticles (SLNs) of duloxetine hydrochloride (DLX) were prepared to circumvent the problems of DLX, which include acid labile nature, high first-pass metabolism, and high-dosing frequency. The DLX-SLNs were prepared by using two different techniques, viz. solvent diffusion method and ultrasound dispersion method, and evaluated for particle size, zeta potential, entrapment efficiency, physical characteristics, and chemical stability. Best results were obtained when SLNs were prepared by ultrasound dispersion method using glyceryl mono stearate as solid lipid and DLX in ratio of 1:20 and mixture of polysorbate 80 and poloxamer 188 as surfactant in concentration of 3%. The mean particle size of formulation and entrapment efficiency was 91.7 nm and 87%, respectively, and had excellent stability in acidic medium. Differential scanning calorimetry and X-ray diffraction data showed complete amorphization of DLX in lipid. In vitro drug release from SLNs was observed for 48 h and was in accordance with Higuchi kinetics. In vivo antidepressant activity was evaluated in mice by forced swim test. DLX-SLNs showed significant enhancement in antidepressant activity at 24 h when administered orally in comparison to drug solution. These results confirm the potential of SLNs in enhancing chemical stability and improving the efficacy of DLX via oral route. The SLN dispersion was converted into solid granules by adsorbing on colloidal silicon dioxide and characterized for particle size after redispersion, morphology, and flow properties. Results indicated that nanoparticles were successfully adsorbed on the carrier and released SLNs when dispersed in water.






Similar content being viewed by others
References
Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25:871–80.
Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40:161–7.
Turcotte JE, Debonnel G, de Montigny C, Hebert C, Blier P. Assessment of the serotonin and norepinephrine reuptake blocking properties of duloxetine in healthy subjects. Neuropsychopharmacology. 2001;24:511–21.
Sinha VR, Kumria R, Bhinge JR. Stress degradation studies on duloxetine hydrochloride and development of an RP-HPLC method for its determination in capsule formulation. J Chromatogr Sci. 2009;47:589–93.
Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59:667–76.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77.
Kumar VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan PV. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335:167–75.
Luo Y, Chen D, Ren L, Zhao X, Qin J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J Control Release. 2006;114:53–9.
Muller RH, Runge S, Ravelli V, Mehnert W, Thunemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317:82–9.
Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS PharmSciTech. 2007;8:24.
Bunjes H, Westesen K, Koch MHJ. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int J Pharm. 1996;129:159–73.
Zimmermann E, Muller RH, Mader K. Influence of different parameters on reconstitution of lyophilized SLN. Int J Pharm. 2000;196:211–3.
Freitas C, Mullera RH. Spray-drying of solid lipid nanoparticles (SLN TM). Eur J Pharm Biopharm. 1998;46:145–51.
Cavalli R, Gasco MR, Barresi AA, Rovero G. Evaporative drying of aqueous dispersions of solid lipid nanoparticles. Drug Dev Ind Pharm. 2001;27:919–24.
Chakraborty S, Shukla D, Vuddanda PR, Mishra B, Singh S. Utilization of adsorption technique in the development of oral delivery system of lipid based nanoparticles. Colloids Surf B Biointerfaces. 2010;81:563–9.
Dixit RP, Nagarsenker MS. Dry adsorbed emulsion of simvastatin: optimization and in vivo advantage. Pharm Dev Technol. 2007;12:495–504.
Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci. 2008;35:183–92.
Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95:627–38.
Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Release. 1999;59:299–307.
Ciulla L, Menezes HS, Bueno BB, Schuh A, Alves RJ, Abegg MP. Antidepressant behavioral effects of duloxetine and fluoxetine in the rat forced swimming test. Acta Cir Bras. 2007;22:351–4.
Li JT, Caldwell KD, Rapoport N. Surface properties of pluronic-coated polymeric colloids. Langmuir. 1994;10:4475–82.
Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf A Physicochem Eng Asp. 2008;315:210–6.
Acknowledgments
The authors are thankful to Shodhna Labs, Hyderabad, India, for providing gift sample of duloxetine hydrochloride and All India Council of Technical Education for funding the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, K., Padhye, S. & Nagarsenker, M. Duloxetine HCl Lipid Nanoparticles: Preparation, Characterization, and Dosage Form Design. AAPS PharmSciTech 13, 125–133 (2012). https://doi.org/10.1208/s12249-011-9727-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9727-6