Abstract
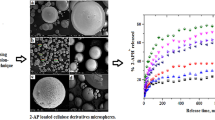
The purpose of the present investigation was to encapsulate pure prednisolone (PRD) and PRD–hydroxypropyl-β-cyclodextrin (HPβCD) complex in cellulose-based matrix microspheres. The system simultaneously exploits complexation technique to enhance the solubility of low-solubility drug (pure PRD) and subsequent modulation of drug release from microspheres (MIC) at a predetermined time. The microspheres of various compositions were prepared by an oil-in-oil emulsion–solvent evaporation method. The effect of complexation and presence of cellulose polymers on entrapment efficiency, particle size, and drug release had been investigated. The solid-state characterization was performed by Fourier transform infrared spectroscopy, thermogravimetry, differential scanning calorimetry, and powder X-ray diffractometry. The morphology of MIC was examined by scanning electron microscopy. The in vitro drug release profiles from these microspheres showed the desired biphasic release behavior. After enhancing the solubility of prednisolone by inclusion into HPβCD, the drug release was easily modified in the microsphere formulation. It was also demonstrated that the CDs in these microspheres were able to modulate several properties such as morphology, drug loading, and release properties. The release kinetics of prednisolone from microspheres followed quasi-Fickian and first-order release mechanisms. In addition to this, the f 2-metric technique was used to check the equivalency of dissolution profiles of the optimized formulation before and after stability studies, and it was found to be similar. A good outcome, matrix microspheres (coded as MIC5) containing PRD–HPβCD complex, showed sustained release of drug (95.81%) over a period of 24 h.










Similar content being viewed by others
REFERENCES
Vijayalakshmi P, Devi VK, Narendra C, Srinagesh S. Development of extended zero-order release gliclazide tablets by central composite design. Drug Dev Ind Pharm. 2008;34:33–45.
Lee DW, Hwang SJ, Park JB, Park HJ. Preparation and release characteristics of polymer-coated and blended alginate microspheres. J Microencapsul. 2003;20:179–92.
Mukherjee B, Santra K, Pattnaik G, Ghosh S. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int J Nanomedicine. 2008;3:487–96.
Trapani A, Laquintana V, Denora N, Lopedota A, Cutrignelli A, Franco M, et al. Eudragit RS 100 microparticles containing 2-hydroxypropyl-β-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur J Pharm Sci. 2007;30:64–74.
Teshima M, Fumoto S, Nishida K, Nakamura J, Ohyama K, Nakamura T, et al. Prolonged blood concentration of prednisolone after intravenous injection of liposomal palmitoyl prednisolone. J Control Release. 2006;112:320–8.
Kramer J, Blume H. Biopharmaceutical aspects of multiparticulates. In: Ghebre-Sellasie Y, editor. Multiparticulate oral drug delivery. New York: Dekker; 1994. p. 307–32.
Guyot M, Fawaz F. Nifedipine loaded-polymeric microspheres: preparation and physical characteristics. Int J Pharm. 1998;175:61–74.
Choudhury PK, Kar M. Controlled release metformin hydrochloride microspheres of ethyl cellulose prepared by different methods and study on the polymer affected parameters. J Microencapsul. 2009;26:46–53.
Akbuga J. Furosemide-loaded ethyl cellulose microspheres prepared by spherical crystallization technique: morphology and release characteristics. Int J Pharm. 1991;76:193–8.
Comoglu T, Gonul N, Dogan A, Basci N. Development and in vitro evaluation of pantoprazole-loaded microspheres. Drug Deliv. 2008;15:295–302.
Bibby DC, Davies NM, Tucker IG. Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int J Pharm. 2000;197:1–11.
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123:78–99.
Fernandes CM, Vieira MT, Veigaa FJB. Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur J Pharm Sci. 2002;15:79–88.
Mura P, Faucci MT, Bettinetti GP. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2001;13:187–94.
Vogt M, Derendorf H, Krämer J, Junginger HE, Midha KK, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: prednisolone. J Pharm Sci. 2007;96:27–37.
Fukuda N, Higuchi N, Ohno M, Kenmochi H, Sekikawa H, Takada M. Dissolution behavior of prednisolone from solid dispersion systems with cyclodextrins and polyvinylpyrrolidone. Chem Pharm Bull. 1986;34:1366–9.
Metselaar JM, Wauben MH, Hilbers JPW, Boerman OC, Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–66.
Schmidt J, Metselaar JM, Wauben MH, Toyka KV, Storm G, Gold R. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain. 2003;126:1895–904.
Berthold A, Cremer K, Kreuter J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J Control Release. 1996;39:17–25.
Oosegi T, Onishi H, Machida Y. Gastrointestinal distribution and absorption behavior of eudragit-coated chitosan–prednisolone conjugate microspheres in rats with TNBS-induced colitis. Int J Pharm. 2008;348:80–8.
Redmon MP, Hickey AJ, Deluca PP. Prednisolone-21-acetate poly(glycolic acid) microspheres: influence of matrix characteristics on release. J Control Release. 1989;9:99–109.
Akiyama Y, Yoshioka M, Horibe H, Hirai S, Kitamori N, Toguchi H. Mechanism of drug release from polyglycerol ester of fatty acid-based microspheres. J Control Release. 1993;27:31–45.
Herrmann J, Bodmeier R. Biodegradable, somatostatin acetate containing microspheres prepared by various aqueous and non-aqueous solvent evaporation methods. Eur J Pharm Biopharm. 1998;45:75–82.
Indian Pharmacopoeia. Published by the Government of India. 2007;II:1584.
Xu GJ, Sunada H. Influence of formulation changes on drug release kinetics from hydroxypropyl methylcellulose matrix tablets. Chem Pharm Bull. 1995;43:483–7.
Singla AK, Medirata DK. Influence of sodium lauryl sulfate on indomethacin release patterns from zinc–indomethacin complex and Indomethacin capsules. Drug Dev Ind Pharm. 1988;14:1883–8.
Higuchi T. Mechanisms of rate of sustained-action medication. J Pharm Sci. 1963;52:1145–9.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Tech. 1996;20:64–75.
Izumikawa S, Yoshioka S, Aso Y, Takeda Y. Preparation of poly(l-lactide) microspheres of different crystalline morphology on drug release rate. J Control Release. 1991;15:133–40.
Palanisamy M, Khanam J, Arunkumar N, Rani C. Design and in vitro evaluation of poly(ε-caprolactone) microspheres containing metoprolol succinate. Asian J Pharm Sci. 2009;4:121–31.
Kakish HF, Tashtoush B, Ibrahim HG, Najib NM. A novel approach for the preparation of highly loaded polymeric controlled release dosage forms of diltiazem HCl and diclofenac sodium. Eur J Pharm Biopharm. 2002;54:75–81.
Yuksel N, Tincer T, Baykara T. Interaction between nicardipine hydrochloride and polymeric microspheres for a controlled release system. Int J Pharm. 1996;140:145–54.
Mishra B, Bansal A, Sankar C. Development and in vitro evaluation of hydrophilic matrix tablets of diltiazem hydrochloride. Acta Pharm Turc. 2005;47:115–26.
Junior AADS, Matos JRD, Formariz TP, Rossanezi G, Scarpa MV, Egito ESTD, et al. Thermal behavior and stability of biodegradable spray-dried microparticles containing triamcinolone. Int J Pharm. 2009;368:45–55.
Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282:1–18.
Maestrelli F, Zerrouk N, Cirri M, Mennini N, Mura P. Microspheres for colonic delivery of ketoprofen–hydroxypropyl-β-cyclodextrin complex. Eur J Pharm Sci. 2008;34:1–11.
Maitre MM, Longhi MR, Granero GG. Ternary complexes of flurbiprofen with HP-β-CD and ethanol amines characterization and transdermal delivery. Drug Dev Ind Pharm. 2007;33:311–26.
Corti G, Capasso G, Maestrelli F, Cirri M, Mura P. Physical–chemical characterization of binary systems of metformin hydrochloride with triacetyl-β-cyclodextrin. J Pharm Biomed Anal. 2007;45:480–6.
Kohata S, Jyodi K, Ohyoshi A. Thermal decomposition of cyclodextrins (α-, β-, γ-, and modified β-CyD) and of metal–(β-CyD) complexes in the solid phase. Thermochim Acta. 1993;217:187–98.
Huang YY, Chung TW, Tzeng TW. A method using biodegradable polylactides/polyethylene glycol for drug release with reduced initial burst. Int J Pharm. 1999;182:93–100.
Kilicarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm. 2003;252:99–109.
Jeyanthi R, Thanoo BC, Metha RC, DeLuca PP. Effect of solvent removal technique on the matrix characteristics of polylactide/glcolide microspheres for peptide delivery. J Control Release. 1996;38:235–44.
Lopedota A, Cutrignelli A, Trapani A, Boghetich G, Denora N, Laquintana V, et al. Effects of different cyclodextrins on the morphology, loading and release properties of poly(dl-lactide-co-glycolide) microparticles containing the hypnotic agent etizolam. J Microencapsul. 2007;24:214–24.
ACKNOWLEDGMENT
We express gratitude to Medopharm, Karnataka, India, for providing prednisolone as gift sample. We wish to thank Signet Chemical Corporation Pvt. Ltd, (Mumbai, India) and Colorcon Asia Pvt. Ltd (Gao, India) for providing polymers free of cost.
Declaration of interest
The authors are thankful to University Grants Commission (UGC), New Delhi, India, for financial support through grant project no. 34–132\2008 (SR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palanisamy, M., Khanam, J. Cellulose-Based Matrix Microspheres of Prednisolone Inclusion Complex: Preparation and Characterization. AAPS PharmSciTech 12, 388–400 (2011). https://doi.org/10.1208/s12249-011-9602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9602-5