Abstract
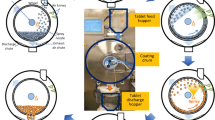
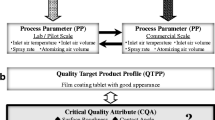
The aim of this study was to identify and optimize the critical process parameters of the newly developed Supercell quasi-continuous coater for optimal tablet coat quality. Design of experiments, aided by multivariate analysis techniques, was used to quantify the effects of various coating process conditions and their interactions on the quality of film-coated tablets. The process parameters varied included batch size, inlet temperature, atomizing pressure, plenum pressure, spray rate and coating level. An initial screening stage was carried out using a 26−1(IV) fractional factorial design. Following these preliminary experiments, optimization study was carried out using the Box–Behnken design. Main response variables measured included drug-loading efficiency, coat thickness variation, and the extent of tablet damage. Apparent optimum conditions were determined by using response surface plots. The process parameters exerted various effects on the different response variables. Hence, trade-offs between individual optima were necessary to obtain the best compromised set of conditions. The adequacy of the optimized process conditions in meeting the combined goals for all responses was indicated by the composite desirability value. By using response surface methodology and optimization, coating conditions which produced coated tablets of high drug-loading efficiency, low incidences of tablet damage and low coat thickness variation were defined. Optimal conditions were found to vary over a large spectrum when different responses were considered. Changes in processing parameters across the design space did not result in drastic changes to coat quality, thereby demonstrating robustness in the Supercell coating process.







Similar content being viewed by others
REFERENCES
Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004;30(5):429–48.
Cerea M, Zheng WJ, Young CR, McGinity JW. A novel powder coating process for attaining taste masking and moisture protective films applied to tablets. Int J Pharm. 2004;279(1–2):127–39.
Ohmori S, Ohno Y, Makino T, Kashihara T. Application of an electronic nose system for evaluation of unpleasant odor in coated tablets. Eur J Pharm Biopharm. 2005;59(2):289–97.
Bechard SR, Quraishi O, Kwong E. Film coating: effect of titanium dioxide concentration and film thickness on the photostability of nifedipine. Pharm Res. 1992;9(10 Suppl):S148.
Pearnchob N, Siepmann J, Bodmeier R. Pharmaceutical applications of shellac: moisture-protective and taste-masking coatings and extended-release matrix tablets. Drug Dev Ind Pharm. 2003;29(8):925–38.
Cole GC. Introduction and overview of pharmaceutical coating. In: Cole GC, editor. Pharmaceutical coating technology. London, UK: Taylor and Francis; 1995. p. 1–5.
Sinha VR, Singh A, Singh S, Bhinge JR. Compression coated systems for colonic delivery of 5-fluorouracil. J Pharm Pharmacol. 2007;59(3):359–65.
Sawada T, Kondo H, Nakashima H, Sako K, Hayashi M. Time-release compression-coated core tablet containing nifedipine for chronopharmacotherapy. Int J Pharm. 2004;280(1–2):103–11.
Porter SC. Coating of tablets and multiparticulates. In: Aulton ME, editor. Aulton’s pharmaceutics: the design and manufacture of medicines. 3rd ed. London, UK: Churchill Livingstone; 2007. p. 500–14.
Marvola M, Rajaniemi M, Marttila E, Vahervuo K, Sothmann A. Effect of dosage form and formulation factors on the adherence of drugs to the esophagus. J Pharm Sci. 1983;7:259–67.
Overgaard ABA, Hojsted J, Hansen R, Moller-Sonnergaard J, Christrup LL. Patients’ evaluation of shape, size and color of solid dosage forms. Pharm World Sci. 2001;23(5):185–8.
Birkmire AP. A novel process for coating objects 3–35 mm in diameter. 11th International Coating Science and Technology Symposium 2004.
Birkmire AP, Liew CV. Tablet coating in the novel Supercell coater: Evaluation of colour uniformity. American Association of Pharmaceutical Scientists Annual Meeting 2004 Poster Session 2004.
Birkmire AP, Liew CV. An accurate method of coating tablets with active pharmaceutical ingredients. 5th European Coating Symposium 2003 Proceedings. 2003:279–84.
Heinamaki J, Ruotsalainen M, Lehtola V, Antikainen O, Yliruusi J. Optimization of aqueous-based film coating of tablets performed by a side-vented pan coating system. Pharm Dev Technol. 1997;2(4):357–64.
Esbensen KH. Glossary of terms. In: Esbensen KH, editor. Multivariate data analysis—in practice. 5th ed. Norway: CAMO; 2001. p. 549–86.
Porter SC, Verseput RP, Cunningham CR. Process optimization using design of experiments. Pharmaceutical Technology Europe. 1997:44–52
Tang ESK, Liew CV, Er DZL, Liu XH, Wigmore AJ, Heng PWS. Study of coat quality of tablets coated by an on-line supercell coater. AAPS PharmSciTech. 2007;8(3):7.
Shelukar S, Ho J, Zega J, Roland E, Yeh N, Quiram D, et al. Identification and characterization of factors controlling tablet coating uniformity in a Wurster coating process. Powder Technol. 2000;110(1–2):29–36.
Picker KM. Time dependence of elastic recovery for characterization of tableting materials. Pharm Dev Technol. 2001;6(1):61–70.
Okutgen E, Hogan JE, Aulton ME. Effects of tablet core dimensional instability on the generation of internal stresses within film coats.3. Exposure to temperatures and relative humidities which mimic the film coating process. Drug Dev Ind Pharm. 1991;17(14):2005–16.
KuShaari K, Pandey P, Song YX, Turton R. Monte Carlo simulations to determine coating uniformity in a Wurster fluidized bed coating process. Powder Technol. 2006;166(2):81–90.
Prater DA, Meakin BJ, Rowe RC, Wilde JS. A technique for investigating changes in the surface roughness of tablets during film coating. J Pharm Pharmacol. 1981;33:666–8.
Perez-Ramos JD, Findlay WP, Peck G, Morris KR. Quantitative analysis of film coating in a pan coater based on in-line sensor measurements. Aaps Pharmscitech. 2005;6(1):E127–36.
Rege BD, Gawel J, Kou JH. Identification of critical process variables for coating actives onto tablets via statistically designed experiments. Int J Pharm. 2002;237(1–2):87–94.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to GEA Pharma Systems, UK, for providing the Supercell coater used in this study. CAMO Softwares, Norway, is also acknowledged for providing The Unscrambler software used for multivariate analysis. Lastly, the authors would like to thank National University of Singapore for the facilities and funding given to this research (Grant No.: R148-000-008-001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cahyadi, C., Heng, P.W.S. & Chan, L.W. Optimization of Process Parameters for a Quasi-Continuous Tablet Coating System Using Design of Experiments. AAPS PharmSciTech 12, 119–131 (2011). https://doi.org/10.1208/s12249-010-9567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9567-9