Abstract
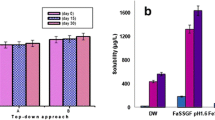
The low water-solubility of gliclazide (GL) leads to a low dissolution rate and variable bioavailability. The aim of this study was to investigate the effect of micronization on the absorption and pharmacokinetics of GL after oral administration in rats. GL microcrystals were prepared using solvent-change and pH-shift methods. Scanning electron microscopy showed considerable changes in the shape and size of crystals using both methods. In the optimized formulation of each method, the particle size of treated GL was reduced about 30 (from 290 to 9.9 μm) and 61 times (to 4.76 μm) by solvent-change and pH-shift methods, respectively. Recrystallized samples showed faster dissolution rate than untreated GL particles. Glucose-lowering effect, C max, and area under the drug concentration-time profile (area under the curve (AUC)) were compared in diabetic and normal rats. AUC and C max were increased by microcrystals in both groups of animals. Administration of 40 mg/kg of GL in the form of untreated drug and microcrystals obtained by solvent-change and pH-shift methods caused 12.49% and 21.04% enhancement in glucose-lowering effect of GL in diabetic rats, respectively.





Similar content being viewed by others
References
Orienti I, Bigucci F, Luppi B, Cerchiara T, Zuccari G, Giunchedi P et al. Polyvinylalcohol substituted with triethyleneglycolmonoethylether as a new material for preparation of solid dispersion of hydrophobic drugs. Eur J Pharm Biopharm. 2002;54:229–33.
Lobenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50:3–12.
Sarkari M, Brown J, Chen X, Swinnea S, William RO, Johnston KP. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int J Pharm. 2002;243:17–31.
Gibson M. Pharmaceutical preformulation and formulation—a practical guide from candidate drug selection to commercial dosage form. Englewood: HIS Health Group; 2001.
Kim ST, Kwon JH, Lee JJ, Kim CW. Microcrystallization of indomethacin using a pH-shift method. Int J Pharm. 2003;263(1–2):141–50.
Chaumeil JC. Micronization: a method of improving the bioavailability of poorly soluble drugs. Meth Find Exp Clin Pharmacol. 1998;20:211–5.
Rasenack N, Muller BW. Dissolution rate enhancement by in situ micronization of poorly water-soluble drugs. Pharm Res. 2002;19(12):1894–900.
Steckel H, Rasenack N, Muller BW. In situ micronization of disodiumcromoglycate for pulmonary delivery. Eur J Pharm Biopharm. 2003;55:173–80.
Williams III RO, Brown J, Liu J. Influence of micronization method on the performance of a suspension triamcinolone acetonide pressurized metered-dose inhaler formulation. Pharm Dev Tech. 1999;4(2):167–79.
Huang QP, Wang JX, Chen GZ, Shen ZG, Chen JF, Yun J. Micronization of gemfibrozil by reactive precipitation process. Int J Pharm. 2008;360:58–64.
Zhang HX, Wang JX, Zhang ZB, Le Y, Shen ZG, Chen JF. Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm. 2009;374(1–2):106–13.
Giry K, Pean JM, Giraud L, Marsas S, Rolland H, Wuthrich P. Drug/lactose co-micronization by jet milling to improve aerosolization properties of a powder for inhalation. Int J Pharm. 2006;321:162–6.
Moribe K, Tsutsumi S, Morishita S, Shinozaki H, Tozuka Y, Oguchi T et al. Micronization of phenylbutazone by rapid expansion of supercritical CO2 solution. Chem Pharm Bull. 2005;53(8):1025–8.
Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophylization techniques. Int J Pharm. 1995;126:155–60.
Palmer KJ, Brogden RN. Gliclazide. An update of its pharmacological properties and therapeutic efficacy in non-insulin-dependent diabetes mellitus. Drugs. 1993;46:93–125.
Arias-Blanco MJ, Moyano JR, Perez-Martinez JI, Gines JM. Study of the inclusion of GL in α-cyclodextrin. J Pharm Biomed Anal. 1998;18:275–9.
Ozkan Y, Atay T, Dikmen N, Isimer A, Aboul-Enein HY. Improvement of water solubility and in vitro dissolution rate of GL by complexation with β-cyclodextrin. Pharm Act Helv. 2000;74:365–70.
Aggarwal S, Singh PN, Mishra B. Studies on solubility and hypoglycemic activity of gliclazide beta-cyclodextrin-hydroxypropylmethylcellulose complexes. Pharmazie. 2002;57(3):191–3.
Moyano JR, Arias-Blanco MJ, Gines JM, Rabasco AM, Perez-Martinez JI, Mor M et al. Nuclear magnetic resonance investigation of the inclusion complexation of GL with β-cyclodextrin. J Pharm Sci. 1997;86(1):72–5.
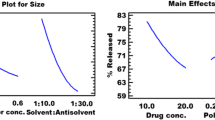
Varshosaz J, Talari R, Mostafavi A, Nokhodchi A. Dissolution enhancement of gliclazide using in situ micronization by solvent change method. Powder Tech. 2008;187:222–30.
Talari R, Varshosaz J, Mostafavi A, Nokhodchi A. Dissolution enhancement of gliclazide using pH change approach in Presence of Twelve Stabilizers with Various Physico-Chemical Properties. J Pharm Pharmaceutic Sci. 2009;12(3):250–65.
Rouini MR, Mohajer A, Tahami MH. A simple and sensitive HPLC method for determination of gliclazide in human serum. J Chromatography B. 2003;785:383–6.
Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. Leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123(3):392–6.
Weiss J, Taylor GR, Zimmermann F, Nebendahl K. Collection of body fluids. In: Krinke GJ, editor. The handbook of experimental animals, the laboratory rat. San Diego: Academic; 2000. p. 488–9.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker; 1982. p. 149–51.
Pandikumar P, Prakash Babu N, Ignacimuthu S. Hypoglycemic and antihyperglycemic effect of Begonia malabarica Lam. in normal and streptozotocin induced diabetic rats. J Ethnopharmacol. 2009;124:111–5.
Kuo CY, Wu SM. High-performance liquid chromatography with electrochemical detection for analysis of gliclazide in plasma. J Chromatogr A. 2005;1088:131–5.
Poirier JM, Perez M, Cheymol G. High-performance liquid chromatographic determination of gliclazide in human plasma. J Chromatogr. 1987;421:223–6.
Charles BG, Ravenscroft PJ. Measurement of gliclazide in plasma by radial compression reversed-phase liquid chromatography. Clin Chem. 1984;30:1789–91.
Yu NH, Ho ENM, Tang FPW, Wan TSM, Wong ASY. Comprehensive screening of acidic and neutral drugs in equine plasma by liquid chromatography–tandem mass spectrometry. J Chrom A. 2008;1189:426–34.
Varshosaz J, Tavakoli N, Minayian M, Rahdari N. Applying the Taguchi design for optimized formulation of sustained-release gliclazide chitosan beads: an in vitro/in vivo study. AAPS Pharm Sci Tech. 2009;10(1):158–65.
Tanira MOM, Furman BL. The in vivo interaction between gliclazide and glibenclamide and insulin on glucose disposal in the rat. Pharmacol Res. 1999;39:349–56.
Gholamhoseinian A, Fallah H, Sharififar F. Inhibitory effect of methanol extract of Rosa damascena Mill. Flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16(10):935–41.
Cunha WR, Arantes GM, Ferreira DS, Lucarini R, Silva MLA, Furtado NAJC et al. Hypoglycemic effect of Leandra lacunosa in normal and alloxan-induced diabetic rats. Fitoterapia. 2008;79:356–60.
Acknowledgment
This work was supported by the Vice Chancellor of Research of Isfahan University of Medical Sciences for financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talari, R., Varshosaz, J., Mostafavi, S.A. et al. Gliclazide Microcrystals Prepared by Two Methods of In Situ Micronization: Pharmacokinetic Studies in Diabetic and Normal Rats. AAPS PharmSciTech 11, 786–792 (2010). https://doi.org/10.1208/s12249-010-9441-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9441-9