Abstract
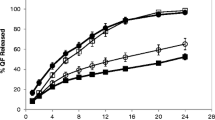
The objective of the present study was to develop a once-daily sustained-release (SR) matrix tablet of famotidine. Nine different formulations (F1–F9) were prepared by direct compression method using Avicel PH101 as filler/binder in the range of 41–27% in F1–F3, 18–22% in F4–F7, and 16–18% in F8–F9 and hydroxypropyl methylcellulose (4,000 cps) as hydrophilic matrix was used in F1–F3 from 19% to 30%, around 40% in F4–F7, and 42–45% in F8–F9. Talc and Aerosil were added in the ratio of 0.7–1.2%. The tablets were subjected to various physical parameters including weight variation test, hardness, thickness, diameter, friability, and in vitro release studies. Assay was also performed according to the USP 30 NF 25 procedure. The results of the physical parameters and assay were found to be within the acceptable range. In vitro dissolution results indicated that formulation F4–F7, having around 40% of rate control polymer, produced a SR pattern throughout 24 h. F1–F3 showed drug release at a faster rate, while F8–F9 released much slower, i.e., <80% in 24 h. Model-dependent and model-independent methods were used for data analysis and the best results were observed for F4 in zero order (r 2 = 0.984) and F6 in Korsmeyer and Higuchi (r 2 = 0.992 and 0.988). The parameter n indicated anomalous diffusion, while β in Weibull showed a parabolic curve with higher initial slope. The f 2 similarity test was performed taking F4 as a reference formulation. Only the F5–F7 formulations were similar to the reference formulation F4. The mean dissolution time was around 10 h for the successful formulation.








Similar content being viewed by others
References
Dollery C. Therapeutic drugs. 2nd ed. Edinburgh: Churchill Livingstone; 1999.
Pepsid. Electronic medicines compendium. Aventis Pharma Ltd. http://emc.medicines.org.uk/emc/assets/c/html/display.doc. Accessed 01 Aug 2003.
Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Technol Prod Manuf. 1984;5:1–9.
Reza MS, Quadir MA, Haid SS. Comparative evaluation of plastic hydrophobic and hydrophilic polymers as matrices for controlled release drug delivery. J Pharm Pharm Sci. 2003;6(2):274–91.
Gothoskar AV. Study of effect of polymer viscosity and polymer: excipient ratio on drug-release patterns from swellable matrices. Drug Delivery Technology. 2005;5:1–7.
VIVAPHARM, cellulose-based polymer for film-coating and sustained-release application. http://www.jrspharma.de/. Accessed 01 Aug 2005.
Rawlins EA. Bentley’s textbook of pharmaceutics. 8th ed. UK: Baillieve Tindall; 1986.
British pharmacopoeia. London: Stationary Office London; 2004.
Gad SC. Pharmaceutical manufacturing handbook. New York: Wiley; 2008.
United States pharmacopoeia 23. Rockville: US Pharmacopeial Convention; 1995.
Hanson WA. Handbook of dissolution testing: compendial method. USA: Pharmaceutical Technology Publication; 1982.
Hadjiioannou TP, Christian GD, Koupparis MA, Macheras PE. Quantitative calculations in pharmaceutical practice and research. New York: VCH; 1993.
Bourne DW. Pharmacokinetics. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker; 2002. p. 66–91.
Higuchi T. Mechanism of sustained action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation theoretical consideration. Ind Eng Chem. 1931;23:923–31.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Langenbucher F. Linearization of dissolution curves by the Weibull distribution. J Pharm Pharmacol. 1972;24:979–81.
Costa P, Sousa Lobo MJ. Modeling and comparison of dissolution profile. Eur J Pharm Sci. 2001;13:123–33.
Baker RW, Lonsdale HS. Controlled release: mechanism and rates. Controlled release of biologically active agents. New York: Plenum; 1974. p. 15–71.
Ritger LP, Peppas NA. A simple equation for description of solute release 1. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42.
Tiwari SB, Rajabi-Siahboomi AR. Applications of complementary polymers in HPMC hydrophilic extended release matrices. Drug Deliv Tech. 2009;9(7):20–7.
Barakat NS, Elbagory IM, Almurshedi AS. Controlled-release carbamazepine matrix granules and tablets comprising lipophilic and hydrophilic components. Drug Deliv. 2009;16(1):57–65.
Tajarobi F, Abrahmsén-Alami S, Hansen M, Larsson A. The impact of dose and solubility of additives on the release from HPMC matrix tablets—identifying critical conditions. Pharm Res. 2009;26(6):1496–503.
Dow Excipients. Technical presentations. http://www.dow.com/dowexcipients/resources/product/methocel/meth_presentations.htm (2009). Accessed 31 May 2009.
Saravanan M, Nataraj KS, Ganesh KS. Hydroxypropyl methyl cellulose based cephalexin extended release tablets: influence of tablet formulation, hardness and storage on in vitro release kinetics. Chem Pharm Bull. 2003;51(8):978–83.
Bravo SA, Lamas MA, Salomon CJ. In-vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J Pharm Sci. 2002;5(3):213–9.
Talukdar MM, Plaizier-vercammen J. Evaluation of xanthan gum as a hydrophilic matrix for controlled release dosage forms preparations. Drug Dev Ind Pharm. 1993;19:1037–46.
Shato H, Miyagawa Y, Okabe T, Miyajima M, Sunada H. Dissolution mechanism of diclofenac sodium from wax matrix granules. J Pharm Sci. 1997;86(8):929–34.
Hakim M. Evaluation of a new rate retarding polymer Kollidon SR as matrix tablets. M.Pharm. thesis, Bangladesh, DU; 2001.
Patel N, Chotai N, Patel J, Soni T, Desai J, Patel R. Comparison of in-vitro dissolution profiles of oxcarbazepine-HP β-CD tablet formulations with marketed oxcarbazepine tablets. http://www.dissolutiontech.com (2008). Accessed 18 Jan 2009.
Karmakar AB, Gonjari ID, Hosmani AH, Dhabale PN, Bhise SB. Dissolution rate enhancement of fenofibrate using liquisolid tablet technique. Part II: evaluation of in vitro dissolution profile comparison method. Lat Am J Pharm. 2009;28(2):219–25.
Yuksel N, Kanik AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA based model dependent and independent methods. Int J Pharm. 2009;209:56–67.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoaib, M.H., Al Sabah Siddiqi, S., Yousuf, R.I. et al. Development and Evaluation of Hydrophilic Colloid Matrix of Famotidine Tablets. AAPS PharmSciTech 11, 708–718 (2010). https://doi.org/10.1208/s12249-010-9427-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9427-7