Abstract
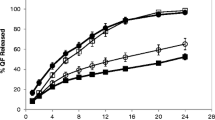
The principles of the percolation theory were applied to further understand and design hydroxypropyl methylcellulose (HPMC) extended release matrix tablets containing carbamazepine and verapamil HCl. This statistical theory studies disordered or chaotic systems where the components are randomly distributed in a lattice. The application of this theory to study the hydration and drug release of hydrophilic matrices allows describing the changes in hydration and drug release kinetics of swellable matrices. The aim of this work was to study and develop extended release matrix formulations for carbamazepine and verapamil HCl, containing hypromellose (HPMC, METHOCEL™ Premium K100M CR) as rate controlling polymer using the concepts of percolation theory. The knowledge of the percolation threshold of the components of the matrix formulations contributes to improve their design. First, reducing the time to market and second, avoiding to formulate in the nearby of the percolation threshold, which will result in a lower variability. Therefore these formulations will be more robust when they are prepared at industrial scale. The HPMC percolation threshold for drugs with very different water solubilities was determined and it was shown that there was no significant influence of drug solubility on the HPMC critical concentration threshold (excipient percolation threshold). This may be related to the versatility and broad functionality of the swelling hydrophilic matrices.




Similar content being viewed by others
References
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel layer behavior, mechanism and optimal performance. Pharm Sci Tech Today. 2000;3:198–204.
Varma M, Kaushal A, Garg A, Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am J Drug Del. 2004;2(1):43–57.
Levina M, Rajabi-Siahboomi AR. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J Pharm Sci. 2004;93(11):2746–54.
Patel DM, Patel NM, Pandya NN, Jogani PD. Gastroretentive drug delivery system of carbamazepine: formulation optimization using simplex lattice design: a technical note. AAPSPharmSciTech. 2007;8(1):Article 11. doi: 10.1208/pt0801011
Passirini N, Perissutti B, Albertini B, Voinovich D, Moneghini M, Rodriguz L. Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J Control Release. 2003;88(2):263–75.
Caraballo I, Millán M, Rabasco AM. Relationship between drug percolation threshold and particle size in matrix tablets. Pharm Res. 1996;13:387–90.
Fuertes I, Miranda A, Millán M, Caraballo I. Estimation of the percolation thresholds in acyclovir hydrophilic matrix tablets. Eur J Pharm Biopharm. 2006;64:336–42.
Miranda A, Millán M, Caraballo I. Study of the critical points in the release and hydration kinetics of swellable matrix type controlled delivery systems. Int J Pharm. 2006;311:75–81.
Miranda A, Millán M, Caraballo I. Study of the critical points in lobenzarit disodium hydrophilic matrices for controlled drug delivery. Chem Pharm Bull. 2006;54(5):598–602.
Miranda A, Millán M, Caraballo I. Investigation of the influence of particle size on the excipient percolation thresholds of HPMC hydrophilic matrix tablets. J Pharm Sci. 2007;96:2745–56.
Gonçalves-Araújo T, Rajabi-Siahboomi AR, Caraballo I. Application of percolation theory in the study of an extended release verapamil hydrochloride formulation. Int J Pharm. 2008;361:112–7.
Leuenberger H, Leu R, Bonny JD. Application of percolation theory and fractal geometry to tablet compaction. Drug Dev Ind Pharm. 1992;18:6723–766.
Ford JL, Mitchell K, Rowe P, Armstrong DJ, Elliot PNC, Rostron C et al. Mathematical modelling of drug release from hydroxypropylmethylcelluose matrices: effect of temperature. Int J Pharm. 1991;71:95–104.
Stauffer D, Aharony A (1992) Introduction to percolation theory, 2nd ed., Francis, London, Washington, DC.
Leuenberger H, Rohera BH, Haas C. Percolation theory—a novel approach to solid dosage form design. Int J Pharm. 1987;38:109–15.
Millán M, Caraballo I, Rabasco A. The role of the drug/excipient particle size ratio in the percolation model for tablets. Pharm Res. 1998;15(2):220–4.
Acknowledgments
The authors wish to express their gratitude to Colorcon for funding this study and for the supply of METHOCEL™ and Recordatti for the supply of the carbamazepine and verapamil HCl.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves-Araújo, T., Rajabi-Siahboomi, A.R. & Caraballo, I. Polymer Percolation Threshold in HPMC Extended Release Formulation of Carbamazepine and Verapamil HCl. AAPS PharmSciTech 11, 558–562 (2010). https://doi.org/10.1208/s12249-010-9408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9408-x