Abstract
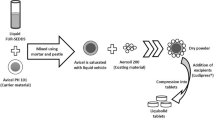
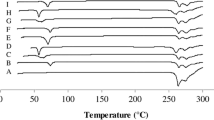
The objective of present investigation was to develop venlafaxine hydrochloride-layered tablets for obtaining sustained drug release. The tablets containing venlafaxine hydrochloride 150 mg were prepared by wet granulation technique using xanthan gum in the middle layer and barrier layers. The granules and tablets were characterized. The in vitro drug dissolution study was conducted in distilled water. The tablets containing two lower strengths were also developed using the same percentage composition of the middle layer. Kinetics of drug release was studied. The optimized batches were tested for water uptake study. Radar diagrams are provided to compare the performance of formulated tablets with the reference products, Effexor XR capsules. The granules ready for compression exhibited good flow and compressibility when xanthan gum was used in the intragranular and extragranular fractions. Monolayer tablets failed to give the release pattern similar to that of the reference product. The drug release was best explained by Weibull model. A unified Weibull equation was evolved to express drug release from the formulated tablets. Lactose facilitated drug release from barrier layers. Substantial water uptake and gelling of xanthan gum appears to be responsible for sustained drug release. The present study underlines the importance of formulation factors in achieving same drug release pattern from three strengths of venlafaxine hydrochloride tablets.







Similar content being viewed by others
References
Lin SY, Lin TL. Different types of direct compressible excipients affecting the release behavior of theophylline controlled-release tablets containing eudragit resins. Drug Dev Ind Pharm 1993;19:1613–21.
Durig T, Fassihi R. Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J Control Rel 2002;80:45–56.
Peppas NA, Gurny R, Doelker E, Buri P. Modelling of drug diffusion through swellable polymeric systems. J Membr Sci 1980;7:241–53.
Lachke A. Xanthan- A Versatile Gum. Resonance 2004;9:25–33.
Talukdar MM, Mooter VD, Augustijns P, Maga TT, Verbeke N, Kinget R. In vitro evaluation of xanthan gum as a potential excipient for oral controlled release matrix tablet formulation. Int J Pharm 1998;169:105–13.
Talukdar MM, Vercammen JP. Evaluation of xanthan gum as a hydrophillic matrix for controlled release dosage forms. Drug Dev Ind Pharm 1993;19:1037–46.
Cox PJ, Khan KA, Munday DL, Sujja-areevath J. Development and evaluation of a multiple-unit oral sustained release dosage form for S(+)-ibuprofen: preparation and release kinetics. Int J Pharm 1999;193:73–84.
Billa N, Yuen KH. Formulation variables affecting drug release from xanthan gum matrices at laboratory scale and pilot scale. AAPS PharmSciTech 2000;1:35–42.
Munday DL, Cox PJ. Compressed xanthan and karaya gum matrices: hydration, erosion and drug release mechanisms. Int J Pharm 2000;203:179–92.
Tobyn MJ, Staniforth JN, Baichwal AR, McCall TW. Prediction of physical properties of a novel polysaccharide controlled release system. Int J Pharm 1996;128:113–22.
Sujja-areevath J, Munday DL, Cox PJ, Khan KA. Relationship between swelling, erosion and drug release in hydrophilic natural gum mini-matrix formulations. Eur J Pharm Sci 1998;6:207–17.
Lu MF, Woodward L, Borodkin S. Xanthan Gum and Alginate Based Controlled Release Theophylline Formulations. Drug Dev Ind Pharm 1991;17:1987–2004.
Dhopeshwarkar V, O’Keeffe JC, Zatz JL, Deete R, Horton M. Development of an Oral Sustained-Release Antibiotic Matrix Tablet Using In Vitro-In Vivo Correlations. Drug Dev Ind Pharm 1994;20:1851–67.
Watanabe K, Yakou S, Takayama K, Machida Y, Nagai T. Factors affecting prednisolone release from hydrogels prepared with water-soluble dietary fibers, xanthan and locust bean gums. Chem Pharm Bull 1992;40:459–62.
Watanabe K, Yakou S, Takayama K, Machida Y, Isowa K, Nagai T. Investigation on rectal absorption of indomethacin from sustained-release hydrogel suppositories prepared with water-soluble dietary fibers, xanthan gum and locust bean gum. Biol Pharm Bull 1993;16:391–4.
Yeole PG, Galgatte UC, Babla IB, Nakhat PD. Design and evaluation of Xanthan gum-based sustained release Matrix tablets of Diclofenac sodium. Indian J Pharm Sci 2006;68:185–9.
Nicholas TW, Jones L, Chamberlain JC. A possible case of venlafaxine-induced Stevens-Johnson syndrome. J Clin Psychiatry 2004;65:1431–2.
Olver JS, Burrows GD, Norman TR. The treatment of depression with different formulations of venlafaxine: a comparative analysis. Hum Psychopharmacol 2004;19:9–16.
Gohel MC, Patel TP, Bariya SH. Studies in preparation and evaluation of pH-independent sustained-release matrix tablets of verapamil HCl using directly compressible eudragits. Pharm Dev Technol 2003;8:323–33.
Costa P. An alternative method to the evaluation of similarity factor in dissolution testing. Int J Pharm 2001;220:77–83.
Higuchi T. Mechanisms of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145–9.
Ertan G, Karasulu HY, Karasulu E, Ege MA, Kose T, Guneri T. A new in vitro/in vivo kinetic correlation method for nitrofurantoin matrix tablet formulations. Drug Dev Ind Pharm 2000;26:737–43.
Langenbucher F. Linearization of dissolution rate curve by the Weibull distribution. J Pharm Pharmacol 1972;24:979–81.
Gohel MC, Parikh RK, Padshala MN, Sarvaiya KG, Jena DG. Formulation and optimization of directly compressible isoniazid modified release matrix tablet. Indian J Pharm Sci 2007;69:640–5.
Roy DS, Rohera BD. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur J Pharm Sci 2002;16:193–9.
Shah VP, Tsong Y, Sathe P, Liu JP. In vitro dissolution profile comparison-statistics and analysis of the similarity factor, f2. Pharm Res 1998;15:889–96.
Shajahan A, Poddar SS. A flexible technology for modified release of drugs: multi layered tablets. J control Rel 2004;97:393–405.
Conte U, Maggi L. Modulation of the dissolution profiles from Geomatrix® multi-layer matrix tablets containing drugs of different solubility. Biomaterials 1996;17:889–96.
Maggi L, Bruni R, Conte U. High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms. Int J Pharm 2000;195:229–38.
Siahi MR, Jalali MB, Monajjemzadeh F, Ghaffari F, Azarmi S. Design and evaluation of 1- and 3-layer matrices of verapamil hydrochloride for sustaining its release. AAPS PharmSciTech 2005;6(4):EE626–EE32. doi:10.1208/pt060477.
El-Nabarawi MA. Modulation of tenoxicam release from hydrophilic matrix: Modulator membrane versus rate-controlling membrane. Chem Pharm Bull 2005;53:1083–7.
FDA Alert for Healthcare Professionals (July 2005): Hydromorphone Hydrochloride Extended-Release Capsules (marketed as Palladone™). http://www.fda.gov/cder/drug/InfoSheets/HCP/hydromorphoneHCP.pdf (accessed 08/03/08), part of http://www.fda.gov (accessed 08/03/08).
Rowe RC, Sheskey PJ, Weller PJ. Xanthan gum. In: Rowe RC, Sheskey PJ, Weller PJ, editors. Handbook of pharmaceutical excipients, 4th edn. London: Pharmaceutical; 2003. p. 691–3.
Dokoumetzidis A, Papadopoulou V, Macheras P. Analysis of dissolution data using modified versions of noyes-whitney equation and the Weibull function. Pharm Res 2006;23:256–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gohel, M.C., Bariya, S.H. Fabrication of Triple-Layer Matrix Tablets of Venlafaxine Hydrochloride Using Xanthan Gum. AAPS PharmSciTech 10, 624–630 (2009). https://doi.org/10.1208/s12249-009-9244-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9244-z