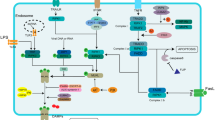
Abstract
Background
Sepsis-induced acute lung injury (ALI) is one of the serious life-threatening complications of sepsis and is pathologically associated with mitochondrial dysfunction. Ginsenoside Rg1 has good therapeutic effects on ALI. Herein, the pharmacological effects of Rg1 in sepsis-induced ALI were investigated.
Methods
Sepsis-induced ALI models were established by CLP operation and LPS treatment. HE staining was adopted to analyze lung pathological changes. The expression and secretion of cytokines were measured by RT-qPCR and ELISA. Cell viability and apoptosis were assessed by MTT assay, flow cytometry and TUNEL staining. ROS level and mitochondrial membrane potential (MMP) were analyzed using DHE probe and JC-1 staining, respectively. FBXO3 m6A level was assessed using MeRIP assay. The interactions between FBXO3, YTHDF1, and PGC-1α were analyzed by Co-IP or RIP.
Results
Rg1 administration ameliorated LPS-induced epithelial cell inflammation, apoptosis, and mitochondrial dysfunction in a dose-dependent manner. Mechanically, Rg1 reduced PGC-1α ubiquitination modification level by inhibiting FBXO3 expression m6A-YTHDF1 dependently. As expected, Rg1’s mitigative effect on LPS-induced inflammation, apoptosis and mitochondrial dysfunction in lung epithelial cells was abolished by FBXO3 overexpression. Moreover, FBXO3 upregulation eliminated the restoring effect of Rg1 on CLP-induced lung injury in rats.
Conclusion
Rg1 activated PGC-1α/Nrf2 signaling pathway by reducing FBXO3 stability in an m6A-YTHDF1-dependent manner to improve mitochondrial function in lung epithelial cells during sepsis-induced ALI progression.
Graphical Abstract








Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- FBXO3:
-
F-Box Protein 3
- PGC:
-
Peroxisome proliferator-activated receptor-γ coactivator
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- MTT:
-
3-(4, 5-Dimethylthiazolyl2)-2, 5-diphenyltetrazolium bromide
- ROS:
-
Reactive oxygen species
- DHE:
-
Dihydroethidium
- ELISA:
-
Enzyme-linked immunosorbent assay
- ATP:
-
Adenosine triphosphate
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- Co-IP:
-
Coimmunoprecipitation
- MeRIP:
-
Methylated RNA immunoprecipitation
- CLP:
-
Cecal ligation and puncture
- HE:
-
Hematoxylin-eosin
- m6A:
-
N6-methyladenosine
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- Bcl-2:
-
B-cell lymphoma-2
- CYCS:
-
Cytochrome C
- NDUFC2:
-
NADH dehydrogenase ubiquinone oxidoreductase subunit C2
- YTHDF1:
-
YTH N6-methyladenosine RNA binding protein 1
References
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51.
Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief. 2013;118:1–8.
Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93(3):329–42.
Gu WJ, Wan YD, Tie HT, Kan QC, Sun TW. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS ONE. 2014;9(2):e90426.
Aziz M, Ode Y, Zhou M, Ochani M, Holodick NE, Rothstein TL, et al. B-1a cells protect mice from sepsis-induced acute lung injury. Mol Med. 2018;24(1):26.
Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28(3):467–95.
Zou Y, Tao T, Tian Y, Zhu J, Cao L, Deng X, et al. Ginsenoside Rg1 improves survival in a murine model of polymicrobial sepsis by suppressing the inflammatory response and apoptosis of lymphocytes. J Surg Res. 2013;183(2):760–6.
Huang L, Cai HA, Zhang MS, Liao RY, Huang X, Hu FD. Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489-3p/Sirt1 axis. J Pharmacol Sci. 2021;147(3):271–83.
Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H, et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121(4):2994–3004.
Ji Q, Sun Z, Yang Z, Zhang W, Ren Y, Chen W, et al. Protective effect of ginsenoside Rg1 on LPS-induced apoptosis of lung epithelial cells. Mol Immunol. 2021;136:168–74.
Wang QL, Yang L, Peng Y, Gao M, Yang MS, Xing W, et al. Ginsenoside Rg1 regulates SIRT1 to ameliorate sepsis-induced lung inflammation and injury via inhibiting endoplasmic reticulum stress and inflammation. Mediators Inflamm. 2019;2019:6453296.
Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 2018;27(3):657-66.e5.
Andrieux P, Chevillard C, Cunha-Neto E, Nunes JPS. Mitochondria as a Cellular Hub in Infection and Inflammation. Int J Mol Sci. 2021;22(21):11338.
Supinski GS, Schroder EA, Callahan LA. Mitochondria and critical illness. Chest. 2020;157(2):310–22.
Kong X, Lin D, Lu L, Lin L, Zhang H, Zhang H. Apelin-13-Mediated AMPK ameliorates endothelial barrier dysfunction in acute lung injury mice via improvement of mitochondrial function and autophagy. Int Immunopharmacol. 2021;101(Pt B):108230.
Johri A, Chandra A, Flint BM. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46.
Li J, Gao W, Zhao Z, Li Y, Yang L, Wei W, et al. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol Neurobiol. 2022;59(5):2855–73.
Liu Z, Pan H, Zhang Y, Zheng Z, Xiao W, Hong X, et al. Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β signaling pathway. J Biochem Mol Toxicol. 2022;36(1):e22885.
Qin Q, Lin N, Huang H, Zhang X, Cao X, Wang Y, et al. Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats. Diabetes Metab Syndr Obes. 2019;12:1091–103.
Gao Y, Xiao X, Luo J, Wang J, Peng Q, Zhao J, et al. E3 ubiquitin ligase FBXO3 drives neuroinflammation to aggravate cerebral ischemia/reperfusion injury. Int J Mol Sci. 2022;23(21):13648.
Mallampalli RK, Coon TA, Glasser JR, Wang C, Dunn SR, Weathington NM, et al. Targeting F box protein Fbxo3 to control cytokine-driven inflammation. J Immunol. 2013;191(10):5247–55.
Chen BB, Coon TA, Glasser JR, McVerry BJ, Zhao J, Zhao Y, et al. A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nat Immunol. 2013;14(5):470–9.
Hung KY, Liao WI, Pao HP, Wu SY, Huang KL, Chu SJ. Targeting F-box protein Fbxo3 attenuates lung injury induced by ischemia-reperfusion in rats. Front Pharmacol. 2019;10:583.
Qian M, Lou Y, Wang Y, Zhang M, Jiang Q, Mo Y, et al. PICK1 deficiency exacerbates sepsis-associated acute lung injury and impairs glutathione synthesis via reduction of xCT. Free Radic Biol Med. 2018;118:23–34.
Zheng F, Wu X, Zhang J, Fu Z, Zhang Y. Sevoflurane reduces lipopolysaccharide-induced apoptosis and pulmonary fibrosis in the RAW264.7 cells and mice models to ameliorate acute lung injury by eliminating oxidative damages. Redox: Rep Commun Free Radic Res. 2022;27(1):139–49.
Liang D, Huang A, Jin Y, Lin M, Xia X, Chen X, et al. Protective effects of exogenous NaHS against sepsis-induced myocardial mitochondrial injury by enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis in mice. Am J Transl Res. 2018;10(5):1422–30.
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48(7):3816–31.
Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376.
Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301.
Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–22.
Mu G, Deng Y, Lu Z, Li X, Chen Y. miR-20b suppresses mitochondrial dysfunction-mediated apoptosis to alleviate hyperoxia-induced acute lung injury by directly targeting MFN1 and MFN2. Acta Biochim Biophys Sin (Shanghai). 2021;53(2):220–8.
Yu T, Tang Y, Zhang F, Zhang L. Roles of ginsenosides in sepsis. J Ginseng Res. 2023;47(1):1–8.
Guo J, Wang R, Min F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J Leukoc Biol. 2022;112(5):1065–77.
Xu M, Ma Q, Fan C, Chen X, Zhang H, Tang M. Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. Int J Mol Sci. 2019;20(23):6086.
Qu M, Chen Z, Qiu Z, Nan K, Wang Y, Shi Y, et al. Neutrophil extracellular traps-triggered impaired autophagic flux via METTL3 underlies sepsis-associated acute lung injury. Cell Death Discov. 2022;8(1):375.
Hu C, Yang L, Wang Y, Zhou S, Luo J, Gu Y. Ginsenoside Rh2 reduces m6A RNA methylation in cancer via the KIF26B-SRF positive feedback loop. J Ginseng Res. 2021;45(6):734–43.
Han Z, Wang X, Xu Z, Cao Y, Gong R, Yu Y, et al. ALKBH5 regulates cardiomyocyte proliferation and heart regeneration by demethylating the mRNA of YTHDF1. Theranostics. 2021;11(6):3000–16.
Zhang S, Guan X, Liu W, Zhu Z, Jin H, Zhu Y, et al. YTHDF1 alleviates sepsis by upregulating WWP1 to induce NLRP3 ubiquitination and inhibit caspase-1-dependent pyroptosis. Cell Death Discov. 2022;8(1):244.
Fontecha-Barriuso M, Martin-Sanchez D, Martinez-Moreno JM, Monsalve M, Ramos AM, Sanchez-Niño MD, et al. The role of PGC-1α and mitochondrial biogenesis in kidney diseases. Biomolecules. 2020;10(2):347.
Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25(4):1354–66.
Chen Q, Shao X, He Y, Lu E, Zhu L, Tang W. Norisoboldine attenuates sepsis-induced acute lung injury by modulating macrophage polarization via PKM2/HIF-1α/PGC-1α pathway. Biol Pharm Bull. 2021;44(10):1536–47.
Funding
This work was supported by Clinical Research Center for Geriatric Diseases of Yunnan Province—diagnosis and treatment of geriatric comorbidity and clinical translational research (202102AA310069), Special Fund of Applied Basic Research of Yunnan Science and Technology Department and Kunming Medical University (202101AY07001-019).
Author information
Authors and Affiliations
Contributions
Rong Liu and Qiang Wang designed this study. Yao Li, Ruixue Wan, Ping Yang, Dexing Yang, Jiefu Tang, and Jiafei Lu collected the materials and performed the experiments. Rong Liu and Qiang Wang analyzed the data and wrote the manuscript. Rong Liu revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All experimental procedures were approved by The First Affiliated Hospital of Kunming Medical University, Yunnan Geriatric Medical Center.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rong Liu and Qiang Wang are the co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Wang, Q., Li, Y. et al. Ginsenoside Rg1 Alleviates Sepsis-Induced Acute Lung Injury by Reducing FBXO3 Stability in an m6A-Dependent Manner to Activate PGC-1α/Nrf2 Signaling Pathway. AAPS J 26, 47 (2024). https://doi.org/10.1208/s12248-024-00919-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-024-00919-5