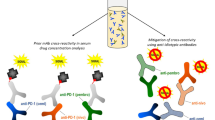
Abstract
The measurement of therapeutic drug concentrations is used to assess drug exposure and the relationship between therapeutic pharmacokinetics (PK) and pharmacodynamics (PD), which help determine the optimal dose for patients. Ligand binding assays (LBAs) are often the method of choice for evaluation of drug concentration and use either the therapeutic target protein or antibodies to the therapeutic as capture and/or detection reagents. Due to the bivalency of antibody therapeutics, heterogeneous states of the drug/target complex can exist in the presence of soluble targets which can complicate measurement of unbound drug. In the case of bispecific antibodies, measurement of drug can be even more complicated and depend upon the levels of both targets to each arm. Measuring the total drug allows for PKPD modeling prediction of human dose projections in addition to overcoming challenges associated with measuring free drug for bispecific antibodies. Here, we present a study in which a sandwich ELISA format was used to measure total anti-KLK5/KLK7 antibody concentrations. This assay utilized a non-blocking anti-idiotype (ID) antibody to one arm of the antibody for capture and an antibody to target bound to the other arm of the antibody for detection. Our qualified assay showed acceptable precision, accuracy, dilutional linearity, and reproducibility and enabled detection of a total bispecific antibody at high levels of two targets. To confirm that our assay was detecting total drug, a subset of samples was evaluated in a generic total LC–MS/MS assay.
Graphical Abstract






Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the study are available upon request.
References
Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. https://doi.org/10.1038/s41573-019-0028-1.
Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314(6012):628–31. https://doi.org/10.1038/314628a0.
Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–65. https://doi.org/10.1016/S1470-2045(22)00335-7.
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47. https://doi.org/10.1056/NEJMoa1609783.
Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2(2):129–36. https://doi.org/10.4161/mabs.2.2.11221.
Vijayaraghavan S, Lipfert L, Chevalier K, Bushey BS, Henley B, Lenhart R, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage Trogocytosis. Mol Cancer Ther. 2020;19(10):2044–56. https://doi.org/10.1158/1535-7163.MCT-20-0071.
Kitazawa T, Esaki K, Tachibana T, Ishii S, Soeda T, Muto A, et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017;117(7):1348–57. https://doi.org/10.1160/TH17-01-0030.
Nicolo M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193–200. https://doi.org/10.1080/13543784.2021.1879791.
Kang J, Sun T, Zhang Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front Immunol. 2022;13:1020003. https://doi.org/10.3389/fimmu.2022.1020003.
Esfandiari A, Cassidy S, Webster RM. Bispecific antibodies in oncology. Nat Rev Drug Discov. 2022;21(6):411–2. https://doi.org/10.1038/d41573-022-00040-2.
Di Paolo CT, Diamandis EP, Prassas I. The role of kallikreins in inflammatory skin disorders and their potential as therapeutic targets. Crit Rev Clin Lab Sci. 2021;58(1):1–16. https://doi.org/10.1080/10408363.2020.1775171.
Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25(2):141–2. https://doi.org/10.1038/75977.
Bellon N, Hadj-Rabia S, Moulin F, Lambe C, Lezmi G, Charbit-Henrion F, et al. The challenging management of a series of 43 infants with Netherton syndrome: unexpected complications and novel mutations. Br J Dermatol. 2021;184(3):532–7. https://doi.org/10.1111/bjd.19265.
Prassas I, Eissa A, Poda G, Diamandis EP. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat Rev Drug Discov. 2015;14(3):183–202. https://doi.org/10.1038/nrd4534.
Kasparek P, Ileninova Z, Zbodakova O, Kanchev I, Benada O, Chalupsky K, et al. KLK5 and KLK7 ablation fully rescues lethality of Netherton syndrome-like phenotype. PLoS Genet. 2017;13(1):e1006566. https://doi.org/10.1371/journal.pgen.1006566.
Chavarria-Smith J, Chiu CPC, Jackman JK, Yin J, Zhang J, Hackney JA, et al. Dual antibody inhibition of KLK5 and KLK7 for Netherton syndrome and atopic dermatitis. Sci Transl Med. 2022;14(675):eabp9159. https://doi.org/10.1126/scitranslmed.abp9159.
Cai H, Tao X, Shim J, Bauer RN, Bremer M, Bu W, et al. Mini-PBPK-based population model and covariate analysis to assess the complex pharmacokinetics and pharmacodynamics of RO7449135, an Anti-KLK5/KLK7 bispecific antibody in cynomolgus monkeys. AAPS J. 2023;25(4):64. https://doi.org/10.1208/s12248-023-00829-y.
Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13(1):99–110. https://doi.org/10.1208/s12248-011-9251-3.
Kuang B, King L, Wang HF. Therapeutic monoclonal antibody concentration monitoring: free or total? Bioanalysis. 2010;2(6):1125–40. https://doi.org/10.4155/bio.10.64.
Fischer SK, Yang J, Anand B, Cowan K, Hendricks R, Li J, et al. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters: case studies. MAbs. 2012;4(5):623–31. https://doi.org/10.4161/mabs.20814.
Talbot JJ, Calamba D, Pai M, Ma M, Thway TM. Measurement of free versus total therapeutic monoclonal antibody in pharmacokinetic assessment is modulated by affinity, incubation time, and bioanalytical platform. AAPS J. 2015;17(6):1446–54. https://doi.org/10.1208/s12248-015-9807-8.
Watanabe H, Shibuya M, Shibahara N, Ruike Y, Sampei Z, Haraya K, et al. A novel total drug assay for quantification of anti-C5 therapeutic monoclonal antibody in the presence of abundant target. AAPS J. 2021;23(1):21. https://doi.org/10.1208/s12248-020-00539-9.
Komatsu N, Saijoh K, Kuk C, Liu AC, Khan S, Shirasaki F, et al. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp Dermatol. 2007;16(6):513–9. https://doi.org/10.1111/j.1600-0625.2007.00562.x.
Komatsu N, Saijoh K, Jayakumar A, Clayman GL, Tohyama M, Suga Y, et al. Correlation between SPINK5 gene mutations and clinical manifestations in Netherton syndrome patients. J Invest Dermatol. 2008;128(5):1148–59. https://doi.org/10.1038/sj.jid.5701153.
Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3(84):84ra44. https://doi.org/10.1126/scitranslmed.3002230.
Ovacik AM, Li J, Lemper M, Danilenko D, Stagg N, Mathieu M, et al. Single cell-produced and in vitro-assembled anti-FcRH5/CD3 T-cell dependent bispecific antibodies have similar in vitro and in vivo properties. MAbs. 2019;11(2):422–33. https://doi.org/10.1080/19420862.2018.1551676.
Ridgway JB, Presta LG, Carter P. “Knobs-into-holes” engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9(7):617–21. https://doi.org/10.1093/protein/9.7.617.
Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;270(1):26–35. https://doi.org/10.1006/jmbi.1997.1116.
Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–9. https://doi.org/10.1038/nbt.1601.
Nnane IP, Han C, Jiao Q, Tam SH, Davis HM, Xu Z. Modification of the Fc region of a human anti-oncostatin M monoclonal antibody for higher affinity to FcRn receptor and extension of half-life in cynomolgus monkeys. Basic Clin Pharmacol Toxicol. 2017;121(1):13–21. https://doi.org/10.1111/bcpt.12761.
Shen Y, Li H, Zhao L, Li G, Chen B, Guo Q, et al. Increased half-life and enhanced potency of Fc-modified human PCSK9 monoclonal antibodies in primates. PLoS One. 2017;12(8):e0183326. https://doi.org/10.1371/journal.pone.0183326.
Saunders KO. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front Immunol. 2019;10:1296. https://doi.org/10.3389/fimmu.2019.01296.
Kaur S, Liu L, Cortes DF, Shao J, Jenkins R, Mylott WR Jr, et al. Validation of a biotherapeutic immunoaffinity-LC-MS/MS assay in monkey serum: “plug-and-play” across seven molecules. Bioanalysis. 2016;8(15):1565–77. https://doi.org/10.4155/bio-2016-0117.
Zhu L, Glick J, Flarakos J. Bioanalytical challenges in support of complex modalities of antibody-based therapeutics. AAPS J. 2020;22(6):130. https://doi.org/10.1208/s12248-020-00517-1.
Wang X, Quarmby V, Ng C, Chuntharapai A, Shek T, Eigenbrot C, et al. Generation and characterization of a unique reagent that recognizes a panel of recombinant human monoclonal antibody therapeutics in the presence of endogenous human IgG. MAbs. 2013;5(4):540–54. https://doi.org/10.4161/mabs.24822.
Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290–6. https://doi.org/10.1038/icb.2014.93.
Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117(17):4542–51. https://doi.org/10.1182/blood-2010-09-306449.
Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001;276(10):7346–50. https://doi.org/10.1074/jbc.M007734200.
Acknowledgements
The authors would like to thank Wei Bu, Jason LaMar, and Rachel Basile for contributions to the mass spectrometry data and Paul Vu and Jose Diaz for providing the critical reagents for our experiments. Manuscript editing was provided by Anshin BioSolutions Corp.
Funding
All work described in this paper was funded by Genentech, Inc. The authors are all employees of Genentech, Inc., and stockholders of the F. Hoffmann-La Roche group.
Author information
Authors and Affiliations
Contributions
J.S.: conceptualization, data curation, formal analysis, methodology, project administration, and roles/writing—original draft; J.C.: data curation, formal analysis, methodology, and qualification; M.C-T.: project administration, writing—review and editing, and supervision; S.K.F.: conceptualization, project administration, writing—review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shim, J., Chen, J., Carrasco-Triguero, M. et al. Overcoming Soluble Target Interference in Measurement of Total Bispecific Therapeutic Antibody Concentrations. AAPS J 25, 82 (2023). https://doi.org/10.1208/s12248-023-00848-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00848-9