Abstract
Macrophages, as one of the most abundant tumor-infiltrating cells, play an important role in tumor development and metastasis. The frequency and polarization of tumor-associated macrophages (TAMs) correlate with disease progression, tumor metastasis, and resistance to various treatments. Pro-inflammatory M1 macrophages hold the potential to engulf tumor cells. In contrast, anti-inflammatory M2 macrophages, which are predominantly present in tumors, potentiate tumor progression and immune escape. Targeting macrophages to modulate the tumor immune microenvironment can ameliorate the tumor-associated immunosuppression and elicit an anti-tumor immune response. Strategies to repolarize TAMs, deplete TAMs, and block inhibitory signaling hold great potential in tumor therapy. Besides, biomimetic carriers based on macrophages have been extensively explored to prolong circulation, enhance tumor-targeted delivery, and reduce the immunogenicity of therapeutics to augment therapeutic efficacy. Moreover, the genetic engineering of macrophages with chimeric antigen receptor (CAR) allows them to recognize tumor antigens and perform tumor cell-specific phagocytosis. These strategies will expand the toolkit for treating tumors, especially for solid tumors, drug-resistant tumors, and metastatic tumors. Herein, we introduce the role of macrophages in tumor progression, summarize the recent advances in macrophage-centered anticancer therapy, and discuss their challenges as well as future applications.

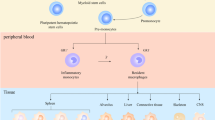
Graphical abstract








Similar content being viewed by others
References
Underhill DM, Gordon S, Imhof BA, Nunez G, Bousso P. Elie Metchnikoff (1845-1916): celebrating 100 years of cellular immunology and beyond. Nat Rev Immunol. 2016;16(10):651–6. https://doi.org/10.1038/nri.2016.89.
Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185(10):2596–606. https://doi.org/10.1016/j.ajpath.2015.06.001.
Gordon S, Pluddemann A, Martinez EF. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262(1):36–55. https://doi.org/10.1111/imr.12223.
Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92. https://doi.org/10.1084/jem.176.1.287.
Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463–88. https://doi.org/10.1615/critrevimmunol.v32.i6.10.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. https://doi.org/10.4049/jimmunol.164.12.6166.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukocyte Biol. 2009;86(5):1065–73. https://doi.org/10.1189/jlb.0609385.
Zhao C, Pang X, Yang Z, Wang S, Deng H, Chen X. Nanomaterials targeting tumor associated macrophages for cancer immunotherapy. J Control Release. 2022;341:272–84. https://doi.org/10.1016/j.jconrel.2021.11.028.
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99(Pt B):180–5. https://doi.org/10.1016/j.addr.2015.11.009.
Zhou XF, Liu XR, Huang L. Macrophage-mediated tumor cell phagocytosis: opportunity for nanomedicine intervention. Adv Funct Mater. 2021;31(5):2006220. https://doi.org/10.1002/adfm.202006220.
Saeed M, Chen F, Ye J, Shi Y, Lammers T, De Geest BG, et al. From design to clinic: engineered nanobiomaterials for immune normalization therapy of cancer. Adv Mater. 2021;33(30):e2008094. https://doi.org/10.1002/adma.202008094.
Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23(8):1148–56. https://doi.org/10.1038/s41590-022-01267-2.
Li CX, Xu XF, Wei SH, Jiang P, Xue LX, Wang JJ. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9(1):e001341. https://doi.org/10.1136/jitc-2020-001341.
Yang QY, Guo NN, Zhou Y, Chen JJ, Wei QC, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharmacol Sin B. 2020;10(11):2156–70. https://doi.org/10.1016/j.apsb.2020.04.004.
Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating beta-catenin/STAT3 signaling. Cell Death Dis. 2020;11(4):234. https://doi.org/10.1038/s41419-020-2435-y.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108(30):12425–30. https://doi.org/10.1073/pnas.1106645108.
Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149(1):36–47. https://doi.org/10.1016/j.cell.2012.03.009.
Garner JM, Fan MY, Yang CH, Du ZY, Sims M, Davidoff AM, et al. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa B signaling in glioblastoma cancer stem cells regulates the notch pathway. J Biolog Chem. 2013;288(36):26167–76. https://doi.org/10.1074/jbc.M113.477950.
Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T, et al. Importance of direct macrophage - tumor cell interaction on progression of human glioma. Cancer Sci. 2012;103(12):2165–72. https://doi.org/10.1111/cas.12015.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin CC, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. https://doi.org/10.1038/nrc3611.
Yang Y, Guo J, Huang L. Tackling TAMs for cancer immunotherapy: it's nano time. Trends Pharmacol Sci. 2020;41(10):701–14. https://doi.org/10.1016/j.tips.2020.08.003.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev Immunol. 2009;9(3):162–74. https://doi.org/10.1038/nri2506.
Geiger R, Rieckmann JC, Wolf T, Basso C, Feng YH, Fuhrer T, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–42. https://doi.org/10.1016/j.cell.2016.09.031.
Horlad H, Ma C, Yano H, Pan C, Ohnishi K, Fujiwara Y, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016;107(11):1696–704. https://doi.org/10.1111/cas.13065.
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37. https://doi.org/10.1084/jem.20082173.
Columba-Cabezas S, Serafini B, Ambrosini E, Sanchez M, Penna G, Adorini L, et al. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J Neuroimmunol. 2002;130(1-2):10–21. https://doi.org/10.1016/S0165-5728(02)00170-4.
Wang D, Yang L, Yue DL, Cao L, Li LF, Wang D, et al. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019;452:244–53. https://doi.org/10.1016/j.canlet.2019.03.040.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nature Rev Drug Discov. 2022;21(11):799–820. https://doi.org/10.1038/s41573-022-00520-5.
Kim IS, Gao Y, Welte T, Wang H, Liu J, Janghorban M, et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. 2019;21(9):1113–26. https://doi.org/10.1038/s41556-019-0373-7.
Koh MY, Sayegh N, Agarwal N. Seeing the forest for the trees-single-cell atlases link CD8(+) T cells and macrophages to disease progression and treatment response in kidney cancer. Cancer Cell. 2021;39(5):594–6. https://doi.org/10.1016/j.ccell.2021.03.008.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–64. https://doi.org/10.1038/s41591-020-1131-x.
Chow A, Schad S, Green MD, Hellmann MD, Allaj V, Ceglia N, et al. Tim-4(+) cavity-resident macrophages impair anti-tumor CD8(+) T cell immunity. Cancer Cell. 2021;39(7):973–88 e9. https://doi.org/10.1016/j.ccell.2021.05.006.
Chen Q, Li YF, Gao WJ, Chen L, Xu WL, Zhu XL. Exosome-mediated crosstalk between tumor and tumor-associated macrophages. Front Mol Biosci. 2021;8:764222. https://doi.org/10.3389/fmolb.2021.764222.
Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31(6):212–9. https://doi.org/10.1016/j.it.2010.04.001.
Zhu S, Yi M, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: implications on therapeutic strategies. Exp Hematol Oncol. 2021;10(1):60. https://doi.org/10.1186/s40164-021-00252-z.
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19(4):541–55. https://doi.org/10.1016/j.ccr.2011.02.006.
Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. https://doi.org/10.1038/nri3789.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–5. https://doi.org/10.1126/science.1252510.
Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 2019;79(4):795–806. https://doi.org/10.1158/0008-5472.CAN-18-2545.
Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–31. https://doi.org/10.1038/nrc2444.
Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–26. https://doi.org/10.1016/j.ccr.2011.02.005.
Tan YF, Wang M, Zhang Y, Ge SY, Zhong F, Xia GW, et al. Tumor-associated macrophages: a potential target for cancer therapy. Front Oncol. 2021;11:693517. https://doi.org/10.3389/fonc.2021.693517.
Uribe-Querol E, Rosales C. Phagocytosis: our current understanding of a universal biological process. Front Immunol. 2020;11:1066. https://doi.org/10.3389/fimmu.2020.01066.
Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–17. https://doi.org/10.1038/ni.3253.
Li H, Somiya M, Kuroda S. Enhancing antibody-dependent cellular phagocytosis by re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials. 2021;268:120601. https://doi.org/10.1016/j.biomaterials.2020.120601.
Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. https://doi.org/10.3389/fimmu.2019.01462.
Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32(40):e2002054. https://doi.org/10.1002/adma.202002054.
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–49. https://doi.org/10.1021/acsnano.7b05465.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59. https://doi.org/10.1016/j.ccr.2014.05.016.
Shen S, Li HJ, Chen KG, Wang YC, Yang XZ, Lian ZX, et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17(6):3822–9. https://doi.org/10.1021/acs.nanolett.7b01193.
Li Z, Ding Y, Liu J, Wang J, Mo F, Wang Y, et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13(1):1845. https://doi.org/10.1038/s41467-022-29388-0.
Zhan X, Jia L, Niu Y, Qi H, Chen X, Zhang Q, et al. Targeted depletion of tumour-associated macrophages by an alendronate-glucomannan conjugate for cancer immunotherapy. Biomat. 2014;35(38):10046–57. https://doi.org/10.1016/j.biomaterials.2014.09.007.
Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7(4):788–99. https://doi.org/10.1158/1535-7163.MCT-07-0579.
Liu XS, Xie XD, Jiang JH, Lin M, Zheng E, Qiu WV, et al. Use of nanoformulation to target macrophages for disease treatment. Adv Funct Mater. 2021;31(38):2104487. https://doi.org/10.1002/adfm.202104487.
Lepland A, Asciutto EK, Malfanti A, Simon-Gracia L, Sidorenko V, Vicent MJ, et al. Targeting pro-tumoral macrophages in early primary and metastatic breast tumors with the CD206-binding mUNO peptide. Mol Pharm. 2020;17(7):2518–31. https://doi.org/10.1021/acs.molpharmaceut.0c00226.
Zang X, Zhang X, Hu H, Qiao M, Zhao X, Deng Y, et al. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol Pharm. 2019;16(5):2249–58. https://doi.org/10.1021/acs.molpharmaceut.9b00261.
Bonavita E, Galdiero MR, Jaillon S, Mantovani A. Phagocytes as corrupted policemen in cancer-related inflammation. Adv Cancer Res. 2015;128:141–71. https://doi.org/10.1016/bs.acr.2015.04.013.
Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587–601. https://doi.org/10.1016/j.ccell.2016.03.005.
Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14(3):155–60. https://doi.org/10.1016/j.semcancer.2003.10.001.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217.
Qian BZ, Li JF, Zhang H, Kitamura T, Zhang JH, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. https://doi.org/10.1038/nature10138.
Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32(1):14–27. https://doi.org/10.1016/j.immuni.2010.01.004.
Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70(12):4840–9. https://doi.org/10.1158/0008-5472.CAN-10-0269.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. https://doi.org/10.1038/s41408-021-00459-7.
Jensen MC, Riddell SR. Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol. 2015;33:9–15. https://doi.org/10.1016/j.coi.2015.01.002.
Zhang C, Zhuang Q, Liu J, Liu X. Synthetic biology in chimeric antigen receptor T (CAR T) cell engineering. ACS Synth Biol. 2022;11(1):1–15. https://doi.org/10.1021/acssynbio.1c00256.
Wang SH, Yang YQ, Ma PW, Zha Y, Zhang J, Lei AH, et al. CAR-macrophage: an extensive immune enhancer to fight cancer. Ebiomedicine. 2022;76:103873. https://doi.org/10.1016/j.ebiom.2022.103873.
Sloas C, Gill S, Klichinsky M. Engineered CAR-macrophages as adoptive immunotherapies for solid tumors. Frontiers in Immunology. 2021;12:783305. https://doi.org/10.3389/fimmu.2021.783305.
Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018;7:e36688. https://doi.org/10.7554/eLife.36688.
Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, et al. CAR-macrophage: a new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021;139:111605. https://doi.org/10.1016/j.biopha.2021.111605.
Villanueva MT. Macrophages get a CAR. Nat Rev Immunol. 2020;20(5):273. https://doi.org/10.1038/s41577-020-0302-9.
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53. https://doi.org/10.1038/s41587-020-0462-y.
Reiss KA, Yuan Y, Ueno NT, Johnson ML, Gill S, Dees EC, et al. A phase 1, first-in-human (FIH) study of the anti-HER2 CAR macrophage CT-0508 in subjects with HER2 overexpressing solid tumors. J Clin Oncol. 2022;40(16):2533. https://doi.org/10.1200/JCO.2022.40.16_suppl.2533.
Chen C, Jing W, Chen Y, Wang G, Abdalla M, Gao L, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. 2022;14(656):eabn1128. https://doi.org/10.1126/scitranslmed.abn1128.
Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2(3):204–15. https://doi.org/10.1159/000296507.
Zheng Y, Han Y, Sun Q, Li Z. Harnessing anti-tumor and tumor-tropism functions of macrophages via nanotechnology for tumor immunotherapy. Exploration (Beijing). 2022;2(3):20210166. https://doi.org/10.1002/EXP.20210166.
Shields CWT, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, et al. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6(18):eaaz6579. https://doi.org/10.1126/sciadv.aaz6579.
Gao C, Wang Q, Li J, Kwong CHT, Wei J, Xie B, et al. In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci Adv. 2022;8(19):eabn1805. https://doi.org/10.1126/sciadv.abn1805.
Cao HQ, Dan ZL, He XY, Zhang ZW, Yu HJ, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. Acs Nano. 2016;10(8):7738–48. https://doi.org/10.1021/acsnano.6b03148.
Shan S, Chen J, Sun Y, Wang Y, Xia B, Tan H, et al. Functionalized macrophage exosomes with panobinostat and PPM1D-siRNA for diffuse intrinsic pontine gliomas therapy. Adv Sci (Weinh). 2022;9(21):e2200353. https://doi.org/10.1002/advs.202200353.
Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34(15):e2110364. https://doi.org/10.1002/adma.202110364.
Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. https://doi.org/10.1016/j.biomaterials.2021.121137.
Hou X, Zhang X, Zhao W, Zeng C, Deng B, McComb DW, et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat Nanotechnol. 2020;15(1):41–6. https://doi.org/10.1038/s41565-019-0600-1.
Li R, He Y, Zhu Y, Jiang L, Zhang S, Qin J, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–34. https://doi.org/10.1021/acs.nanolett.8b03439.
Gao C, Huang Q, Liu C, Kwong CHT, Yue L, Wan JB, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. https://doi.org/10.1038/s41467-020-16439-7.
Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281(1):51–61. https://doi.org/10.1016/j.cellimm.2013.01.010.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. https://doi.org/10.1016/j.it.2004.09.015.
Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):127. https://doi.org/10.1038/s41392-021-00506-6.
Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22(13):6995. https://doi.org/10.3390/ijms22136995.
Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–87. https://doi.org/10.1038/s41423-020-00541-3.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. https://doi.org/10.1038/nrd.2018.169.
Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol. 2017;8:828. https://doi.org/10.3389/fimmu.2017.00828.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. https://doi.org/10.1016/j.ccr.2013.09.014.
He XY, Liu BY, Wu JL, Ai SL, Zhuo RX, Cheng SX. A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression. ACS Appl Mater Interfaces. 2017;9(49):42566–76. https://doi.org/10.1021/acsami.7b13594.
Stanczak MA, Rodrigues Mantuano N, Kirchhammer N, Sanin DE, Jacob F, Coelho R, et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci Transl Med. 2022;14(669):eabj1270. https://doi.org/10.1126/scitranslmed.abj1270.
Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–22. https://doi.org/10.1002/anie.201912524.
Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–108. https://doi.org/10.1021/acsnano.8b05292.
Wang Y, Zhao C, Liu Y, Wang C, Jiang H, Hu Y, et al. Recent advances of tumor therapy based on the CD47-SIRPalpha axis. Mol Pharm. 2022;19(5):1273–93. https://doi.org/10.1021/acs.molpharmaceut.2c00073.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. P Natl Acad Sci USA. 2012;109(17):6662–7. https://doi.org/10.1073/pnas.1121623109.
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110(27):11103–8. https://doi.org/10.1073/pnas.1305569110.
Kurdi AT, Glavey SV, Bezman NA, Jhatakia A, Guerriero JL, Manier S, et al. Antibody-dependent cellular phagocytosis by macrophages is a novel mechanism of action of elotuzumab. Mol Cancer Ther. 2018;17(7):1454–63. https://doi.org/10.1158/1535-7163.Mct-17-0998.
Mantovani A, Caprioli V, Gritti P, Spreafico F. Human mature macrophages mediate antibody-dependent cellular cytotoxicity on tumour cells. Transplantation. 1977;24(4):291–3. https://doi.org/10.1097/00007890-197710000-00010.
Zhang WT, Huang QH, Xiao WW, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRP alpha axis. Front Immunol. 2020;11:18. https://doi.org/10.3389/fimmu.2020.00018.
Bouwstra R, van Meerten T, Bremer E. CD47-SIRP alpha blocking-based immunotherapy: current and prospective therapeutic strategies. Clin Transl Med. 2022;12(8):e943. https://doi.org/10.1002/ctm2.943.
Koenig KL, Borate U. New investigational combinations for higher-risk MDS. Hematol-Am Soc Hemat. 2022;1:368–74. https://doi.org/10.1182/hematology.2022000351.
Cabrales P, Carter C, Oronsky B, Reid T. Rrx-001 is a phase 3 small molecule dual inhibitor of CD47 and Sirp alpha with activity in multiple myeloma. Blood. 2018;132:5623. https://doi.org/10.1182/blood-2018-99-116947.
Huang Y, Ma Y, Gao P, Yao Z. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis. 2017;9(2):E168–E74. https://doi.org/10.21037/jtd.2017.02.30.
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14(1):89–97. https://doi.org/10.1038/s41565-018-0319-4.
Wang F, Huang Q, Su H, Sun M, Wang Z, Chen Z, et al. Self-assembling paclitaxel-mediated stimulation of tumor-associated macrophages for postoperative treatment of glioblastoma. Proc Natl Acad Sci U S A. 2023;120(18):e2204621120. https://doi.org/10.1073/pnas.2204621120.
Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J, et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng. 2018;2(8):589–99. https://doi.org/10.1038/s41551-018-0254-6.
Feng QQ, Ma XT, Cheng KM, Liu GN, Li Y, Yue YL, et al. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater. 2022;34(40):e2206200. https://doi.org/10.1002/adma.202206200.
Wen J, Wang S, Guo R, Liu D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur J Med Chem. 2023;245(Pt 1):114884. https://doi.org/10.1016/j.ejmech.2022.114884.
Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. 2022;21(8):601–20. https://doi.org/10.1038/s41573-022-00470-y.
Bae SH, Kim JH, Park TH, Lee K, Lee BI, Jang H. BMS794833 inhibits macrophage efferocytosis by directly binding to MERTK and inhibiting its activity. Exp Mol Med. 2022;54(9):1450–60. https://doi.org/10.1038/s12276-022-00840-x.
Zhuang WR, Wang Y, Nie W, Lei Y, Liang C, He J, et al. Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity. Nat Commun. 2023;14(1):1675. https://doi.org/10.1038/s41467-023-37369-0.
Komohara Y, Niino D, Ohnishi K, Ohshima K, Takeya M. Role of tumor-associated macrophages in hematological malignancies. Pathol Int. 2015;65(4):170–6. https://doi.org/10.1111/pin.12259.
Saito Y, Komohara Y, Niino D, Horlad H, Ohnishi K, Takeya H, et al. Role of CD204-positive tumor-associated macrophages in adult T-cell leukemia/lymphoMa. J Clin Exp Hematop. 2014;54(1):59–65. https://doi.org/10.3960/jslrt.54.59.
Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M. Contribution of macrophage polarization to metabolic diseases. J Atheroscler Thromb. 2016;23(1):10–7. https://doi.org/10.5551/jat.32359.
Cai H, Zhang Y, Wang J, Gu J. Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front Immunol. 2021;12:690869. https://doi.org/10.3389/fimmu.2021.690869.
Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175(2):442–57 e23. https://doi.org/10.1016/j.cell.2018.09.007.
Acknowledgements
Q. H. acknowledges the start-up package support from the University of Wisconsin-Madison.
Author information
Authors and Affiliations
Contributions
Writing: Y.W. and A.B.
Funding acquisition: Q.H.
Supervision: Q.H.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Responsible Editor: Aliasger Salem
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Barrett, A. & Hu, Q. Targeting Macrophages for Tumor Therapy. AAPS J 25, 80 (2023). https://doi.org/10.1208/s12248-023-00845-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00845-y