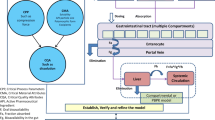
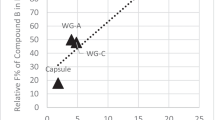
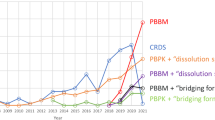
Abstract
Quality risk assessment following ICH Q9 principles is an important activity to ensure optimal clinical efficacy and safety of a drug product. Typically, risk assessment is focused on product performance wherein critical material attributes, formulation variables, and process parameters are evaluated from a manufacturing perspective. Extending ICH Q9 principles to biopharmaceutics risk assessment to identify factors that can impact in vivo performance is an upcoming area. This is evident by recent regulatory trends wherein a new term critical bioavailability attributes (CBA) has been coined to identify such factors. Although significant work has been performed for biopharmaceutics risk assessment for new molecules, there is a need for harmonized biopharmaceutics risk assessment workflow for generic submissions. In this manuscript, we attempted to provide a framework for performing biopharmaceutics risk assessment for generic regulatory submissions. A detailed workflow for performing biopharmaceutics risk assessment includes identification of initial CBA (iCBA), their confirmatory evaluation followed by definition of the control strategy. Tools for biopharmaceutics risk assessment, i.e., bio-discriminatory dissolution method and physiologically based biopharmaceutics modeling (PBBM) were discussed from a practical perspective. Furthermore, a case study for CBA evaluation using PBBM modeling for an extended-release product for regulatory submission has been described using the proposed workflow. Finally, future directions of integrating CBA evaluation, biopharmaceutics risk assessment to the FDA Knowledge Aided Structured Assessment (KASA) initiative, the necessity of risk assessment templates, and knowledge sharing between industry and academia are discussed. Overall, the work described in this manuscript can facilitate and provide guidance for biopharmaceutics risk assessment for generic submissions.
Graphical Abstract







Similar content being viewed by others
Abbreviations
- ANDA:
-
Abbreviated new drug application
- ANVISA:
-
Agência Nacional de Vigilância Sanitária (Brazilian Health Regulatory Agency)
- API:
-
Active pharmaceutical ingredients
- ARA:
-
Acid-reducing agents
- BCS:
-
Biopharmaceutics classification system
- BioRAM:
-
Biopharmaceutics risk assessment road map
- BMR:
-
Batch manufacturing record
- BP:
-
Blueprint
- CBA:
-
Critical bioavailability attributes
- CFV:
-
Critical formulation variables
- CMA:
-
Critical material attributes
- CMC:
-
Chemistry, manufacturing, and controls
- CPP:
-
Critical process variables
- CQA:
-
Critical quality attribute
- CRCG:
-
Center for Research on Complex Generics (CRCG)
- CRDS:
-
Clinically relevant dissolution specifications
- DDDPlus:
-
Dose disintegration and dissolution plus
- DoE:
-
Design of experimentation
- DR:
-
Delayed release
- EMA:
-
European Medicines Agency
- ER:
-
Extended release
- FDA:
-
Food and drug administration
- GDF:
-
Generic drug forum
- iCBA:
-
Initial critical bioavailability attributes
- ICH:
-
International Council for Harmonization
- IR:
-
Immediate release
- IVIVC:
-
in vitro in vivo Correlation
- IVIVR:
-
in vitro in vivo Relation
- KASA:
-
Knowledge Aided Structured Assessment
- MR:
-
Modified release
- NCE:
-
New chemical entity
- NTI:
-
Narrow therapeutic index
- OGD:
-
Office of generic drugs
- PAS:
-
Prior approval supplement
- PBBM:
-
Physiologically based biopharmaceutics model
- PBPK:
-
Physiologically based pharmacokinetic model
- PCQS:
-
Patient centric quality standards
- PK:
-
Pharmacokinetics
- PPI:
-
Proton pump inhibitors
- Q1/Q2:
-
Qualitatively similar/quantitatively similar
- QbD:
-
Quality by design
- QC:
-
Quality control
- QRM:
-
Quality risk management
- QTPP:
-
Quality target product profile
- SBIA:
-
CDER Small Business & Industry Assistance
- SUPAC:
-
Scale-up and post-approval changes
- TGA:
-
Therapeutic goods administration
- USFDA:
-
United States Food and drug administration
References
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25:781–91. https://doi.org/10.1007/s11095-007-9511-1.
ICH Harmonized Tripartite Guideline. Pharmaceutical Development Q8(R2), 2009. https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf. Accessed 02nd Apr 2023.
Zhang L, Mao S. Application of quality by design in the current drug development. Asian J Pharm Sci. 2017;12(1):1–8. https://doi.org/10.1016/j.ajps.2016.07.006.
Guidance for industry. Q10: pharmaceutical quality system. 2009. https://www.fda.gov/media/71553/download. Accessed 02nd Apr 2023.
Flanagan T, Peer AV, Lindahl A. Use of physiologically relevant biopharmaceutics tools within the pharmaceutical industry and in regulatory sciences: where are we now and what are the gaps? Eur J Pharm Sci. 2016;91:84–90. https://doi.org/10.1016/j.ejps.2016.06.006.
Selen A, Dickinson PA, Mullertz A, Crison JR, Mistry HB, Cruanes MT, et al. The biopharmaceutics risk assessment roadmap for optimizing clinical drug product performance. J Pharm Sci. 2014;103:3377–97. https://doi.org/10.1002/jps.24162.
Selen A, Mullertz A, Kesisoglou F, Ho RJY, Cook JA, Dickinson PA, et al. Integrated multi-stakeholder systems thinking strategy: decision-making with biopharmaceutics risk assessment roadmap (BioRAM) to optimize clinical performance of drug products. AAPS J. 2020;22:97. https://doi.org/10.1208/s12248-020-00470-z.
Charoo NA, Cristofoletti R, Kim SK. Integrating biopharmaceutics risk assessment and in vivo absorption model in formulation development of BCS class I drug using the QbD approach. Drug Dev Ind Pharm. 2017;43(4):668–77. https://doi.org/10.1080/03639045.2016.1278013.
Raines K. PBPK biopharmaceutics guidance and progress on risk assessment. Regulatory utility of mechanistic modeling to support alternative bioequivalence approaches workshop. Oct 2021. https://www.complexgenerics.org/media/SOP/complexgenerics/pdf/Conference-Slides/D2-04%20Kimberly%20Raines_PBPKGuidanceRiskAssessment.pdf, Accessed 02nd Apr 2023.
McAllister M, Flanagan T, Cole S, Abend A, Kotzagiorgis E, Limberg J, et al. Developing clinically relevant dissolution specifications (CRDSs) for oral drug products: virtual webinar series. Pharmaceutics. 2022;14(5):1010. https://doi.org/10.3390/pharmaceutics14051010.
Wu D, Sanghavi M, Kollipara S, Ahmed T, Saini AK, Heimbach T. Physiologically based pharmacokinetics modeling in biopharmaceutics: case studies for establishing the bioequivalence safe space for innovator and generic drugs. Pharm Res. 2023;40(2):337–57. https://doi.org/10.1007/s11095-022-03319-6.
USFDA, Guidance for Industry. Scale-up and postapproval changes: chemistry, manufacturing, and controls; in vitro dissolution testing and in vivo bioequivalence documentation. SUPAC-MR. https://www.fda.gov/media/70956/download. Accessed 02nd Apr 2023.
ICH guideline Q9 on quality risk management. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-3.pdf. Accessed 02nd Apr 2023.
EMA, Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. 2019, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf. Accessed 02nd Apr 2023.
USFDA, Guidance for Industry. The use of physiologically based pharmacokinetic analyses – biopharmaceutics applications for oral drug product development, manufacturing changes, and controls, 2020. https://www.fda.gov/media/142500/download. Accessed 02nd Apr 2023.
Fang W. PBPK absorption modeling to support risk assessment and biowaiver for generic oral products. PBPK 2021: Regulatory Utility of Mechanistic Modeling to Support Alternative Bioequivalence Approaches. Oct 2021. https://www.complexgenerics.org/media/SOP/complexgenerics/pdf/Conference-Slides/D2-03%202021_PBPK_workshop_Fang%20Wu_Presentation_Final_Modified_for_Posting.pdf. Accessed 02nd Apr 2023.
Fang W. Using PBPK model to support risk assessment for oral products, from a regulatory perspective. FDA CRCG 2022: best practices for utilizing modeling approaches to support generic product development. Oct 2022. https://www.complexgenerics.org/media/SOP/complexgenerics/pdf/Conference-Slides/Modeling-Approaches/3-1%20Fang%20Wu.pdf. Accessed 02nd Apr 2023.
Rebeka J. PBPK modeling to support risk assessment for oral drug products, including waiver of fed BE studies. Oct 2022. https://www.complexgenerics.org/media/SOP/complexgenerics/pdf/Conference-Slides/Modeling-Approaches/3-2%20Rebeka%20Jereb.pdf. Accessed 02nd Apr 2023.
Om A. Clinically relevant dissolution specifications: a biopharmaceutics’ risk based approach: an FDA perspective. May 2021. https://www.apsgb.co.uk/wp-content/uploads/2021/05/Clinically-Relevant-Dissolution-Specifications-an-FDA-Perspective-__Om-Anand.pdf. Accessed 02nd Apr 2023.
Yu LX, Raw A, Wu L, Capacci-Daniel C, Zhang Y, Rosencrance S. FDA’s new pharmaceutical quality initiative: knowledge-aided assessment & structured applications. Int J Pharm X, 2019;17: 100010.j.ijpx.2019.100010
Raines K. Use of knowledge-aided assessment and structured application (KASA) in biopharmaceutics assessment. SBIA Generic Drug Annual Forum (GDF). April 2022. https://www.fda.gov/media/165528/download. Accessed 02nd Apr 2023.
Grady H, Elder D, Webster GK, Mao Y, Lin Y, Flanagan T, et al. Industry’s view on using quality control, biorelevant, and clinically relevant dissolution tests for pharmaceutical development, registration, and commercialization. J Pharm Sci. 2018;107(1):34–41. https://doi.org/10.1016/j.xphs.2017.10.019.
Al-Tabakha MM, Alomar MJ. In vitro dissolution and in silico modeling shortcuts in bioequivalence testing. Pharmaceutics. 2020;12(1):45. https://doi.org/10.3390/pharmaceutics12010045.
Polli JE, Yu LX, Cook JA, Amidon GL, Borchardt RT, Burnside BA, et al. Summary workshop report: biopharmaceutics classification system—implementation challenges and extension opportunities. J Pharm Sci. 2004;93(6):P1375-1381. https://doi.org/10.1002/jps.20064.
USFDA, Guidance for industry. M9 biopharmaceutics classification system based biowaivers. May 2021.https://www.fda.gov/media/148472/download. Accessed 02nd Apr 2023.
EMA, ICH M9 guideline on biopharmaceutics classification system-based biowaivers. July 2020. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-5_en.pdf. Accessed 02nd Apr 2023.
Kollipara S, Ahmed T, Bhattiprolu AK, Chachad S. In vitro and in silico biopharmaceutic regulatory guidelines for generic bioequivalence for oral products: comparison among various regulatory agencies. Biopharm Drug Dispos. 2021;42(7):297–318. https://doi.org/10.1002/bdd.2292.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The biopharmaceutics classification system: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci. 2014;57:152–63. https://doi.org/10.1016/j.ejps.2014.01.009.
Beg S, Hasnain MS, Rahman M, Swain S. Chapter 1 - introduction to quality by design (QbD): fundamentals, principles, and applications. Pharmaceutical Quality by Design. 2019; 1–17. https://doi.org/10.1016/B978-0-12-815799-2.00001-0.
Yuvaneshwari K, Kollipara S, Ahmed T, Chachad S. Applications of PBPK/PBBM modeling in generic product development: an industry perspective. J Drug Del Sci Technol. 2022;69:103152. https://doi.org/10.1016/j.jddst.2022.103152.
McAllister M, Flanagan T, Boon K, Pepin X, Tistaert C, Jamei M, et al. Developing clinically relevant dissolution specifications for oral drug products—industrial and regulatory perspectives. Pharmaceutics. 2020;12(1):19. https://doi.org/10.3390/pharmaceutics12010019.
Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P et al. Applications of clinically relevant dissolution testing: workshop summary report. AAPS J. 2018;20:93. s12248–018–0252–3.
Hermans A, Abend AM, Kesisoglou F, Flanagan T, Cohen MJ, Diaz DA. Approaches for establishing clinically relevant dissolution specifications for immediate release solid oral dosage forms. AAPS J. 2017;19:1537–49. https://doi.org/10.1208/s12248-017-0117-1.
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, et al. Dissolution and translational modeling strategies toward establishing an in vitro-in vivo link-a workshop summary report. AAPS J. 2019;21(2):29. https://doi.org/10.1208/s12248-019-0298-x.
Paraiso RLM, Rose RH, Fotaki N, McAllister M, Dressman JB. The use of PBPK/PD to establish clinically relevant dissolution specifications for zolpidem immediate release tablets. Eur J Pharm Sci. 2020;115:105534. https://doi.org/10.1016/j.ejps.2020.105534.
Kollipara S, Bhattiprolu AK, Boddu R, Ahmed T, Chachad S. Best practices for integration of dissolution data into physiologically based biopharmaceutics models (PBBM): a biopharmaceutics modeling scientist perspective. AAPS PharmSciTech. 2023;24(2):59. https://doi.org/10.1208/s12249-023-02521-y.
USFDA Dissolution database, https://www.fda.gov/drugs/drug-approvals-and-databases/dissolution-methods-database Accessed 24th May 2023.
Gray VA, Mann JC, Barker R, Pepin XJH. The case for physiologically based biopharmaceutics modelling (PBBM): what do dissolution scientists need to know? Disso Technol. 2020;27(3):6. https://doi.org/10.14227/DT270320P6.
USFDA, Guidance for industry. Dissolution testing and acceptance criteria for immediate-release solid oral dosage form drug products containing high solubility drug substances. Aug 2018. https://www.fda.gov/files/drugs/published/Dissolution-Testing-and-Acceptance-Criteria-for-Immediate-Release-Solid-Oral-Dosage-Form-Drug-Products-Containing-High-Solubility-Drug-Substances-Guidance-for-Industry.pdf. Accessed 02nd Apr 2023.
Bhattiprolu AK, Kollipara S, Ahmed T, Boddu R, Chachad S. Utility of physiologically based biopharmaeutics modeling (PBBM) in regulatory perspective: application to supersede f2, enabling biowaivers & creation of dissolution safe space. J Pharm Sci. 2022;111(12):3397–410. https://doi.org/10.1016/j.xphs.2022.09.003.
Almukainzi M, Okamu A, Wei H, Lobenberg R. Simulation of in vitro dissolution behavior using DDDPlus™. AAPS PharmSciTech. 2015;16(1):217–21. https://doi.org/10.1208/s12249-014-0241-5.
DDDPlusTM, Simulations Plus, https://www.simulations-plus.com/software/dddplus/. Accessed 24th May 2023.
Mitra A, Suarez-Sharp S, Pepin XJH, Flanagan T, Zhao Y, Kotzagiorgis E, Parrott N, et al. Applications of physiologically based biopharmaceutics modeling (PBBM) to support drug product quality: a workshop summary report. J Pharm Sci. 2021;110(2):594–609. https://doi.org/10.1016/j.xphs.2020.10.059.
Jaiswal S, Ahmed T, Kollipara S, Bhargava M, Chachad S. Development, validation and application of physiologically based biopharmaceutics model to justify the change in dissolution specifications for DRL ABC extended release tablets. Drug Dev Ind Pharm. 2021;47(5):778–89. https://doi.org/10.1080/03639045.2021.1934870.
Heimbach T, Kesisoglou F, Novakovic J, Tistaert C, Mueller-Zsigmondy M, Kollipara S, Ahmed T, et al. Establishing the bioequivalence safe space for immediate-release oral dosage forms using physiologically based biopharmaceutics modeling (PBBM): Case Studies. J Pharm Sci. 2021;110(12):3896–906. https://doi.org/10.1016/j.xphs.2021.09.017.
Kesisoglou F. Can PBPK Modeling streamline food effect assessments? J Clin Pharmacol. 2020;60(S1):S98-104. https://doi.org/10.1002/jcph.1678.
Parrott N, Suarez-Sharp S, Kesisoglou F, Pathak SM, Good D, Wagner C et al. Best practices in the development and validation of physiologically based biopharmaceutics modeling. a workshop summary report. J Pharm Sci, 2021;110(2):584–593. https://doi.org/10.1016/j.xphs.2020.09.058.
Kollipara S, Boddu R, Ahmed T, Chachad S. Simplified model-dependent and model-independent approaches for dissolution profile comparison for oral products: regulatory perspective for generic product development. AAPS PharmSciTech. 2022;23(1):53. https://doi.org/10.1208/s12249-021-02203-7.
Anand O, Pepin XJH, Kolhatkar V, Seo P. The use of physiologically based pharmacokinetic analyses-in biopharmaceutics applications -regulatory and industry perspectives. Pharm Res. 2022;39(8):1681–700. https://doi.org/10.1007/s11095-022-03280-4.
Kuemmel C, Yang Y, Zhang X, Florian J, Zhu H, Tegenge M, et al. Consideration of a credibility assessment framework in model-informed drug development: potential application to physiologically-based pharmacokinetic modeling and simulation. CPT Pharmacometrics Syst Pharmacol. 2020;9(1):21–18. https://doi.org/10.1002/psp4.12479.
Acknowledgements
The authors would like to thank Dr. Reddy’s Laboratories Ltd. for providing an opportunity to publish this review article.
Funding
This article is funded by Dr. Reddy’s Laboratories Ltd.
Author information
Authors and Affiliations
Contributions
Tausif Ahmed: concept and design, writing the manuscript, manuscript review, approval for the version to be published; Sivacharan Kollipara: concept and design, writing the manuscript; Rajkumar Boddu: concept and design, writing manuscript; Adithya Karthik Bhattiprolu: concept and design, writing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All the authors are employees of Dr. Reddy’s Laboratories Ltd. and report no conflicts interest. The authors alone are responsible for the content and writing of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, T., Kollipara, S., Boddu, R. et al. Biopharmaceutics Risk Assessment—Connecting Critical Bioavailability Attributes with In Vitro, In Vivo Properties and Physiologically Based Biopharmaceutics Modeling to Enable Generic Regulatory Submissions. AAPS J 25, 77 (2023). https://doi.org/10.1208/s12248-023-00837-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00837-y