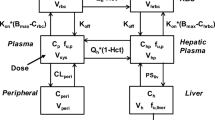
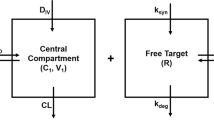
Abstract
In general, small-molecule target-mediated drug disposition (TMDD) is caused by the interaction of a drug with its high-affinity, low-capacity pharmacological target. In the current work, we developed a pharmacometrics model to characterize a new type of TMDD, where the nonlinear pharmacokinetics (PK) is mediated by a high-capacity pharmacological target with cooperative binding instead of target saturation. The model drug we used was PF-07059013, a noncovalent hemoglobin modulator that demonstrated promising preclinical efficacy to treat sickle cell disease (SCD), and showed complex nonlinear PK in mice with the fraction of unbound drug in blood (fub) decreased with an increase in PF-07059013 concentrations/doses due to the positive cooperative binding of PF-07059013 to hemoglobin. Among the various models we evaluated, the best one is a semi-mechanistic model where only drug molecules not bound to hemoglobin were allowed for elimination, with the nonlinear pharmacokinetics being captured by incorporating cooperative binding for drug molecules bound to hemoglobin. Our final model provided valuable insight on target binding-related parameters, such as the Hill coefficient γ (estimated to be 1.6), binding constant KH (estimated to be 1450 µM), and the amount of total hemoglobin Rtot (estimated to be 2.13 µmol). As the dose selection of a compound with positive cooperative binding is tricky and challenging due to the nonproportional and steep response, our model may be valuable in facilitating the rational dose regimen selection for future preclinical animal and clinical trials for PF-07059013 and other compounds whose nonlinear pharmacokinetics are caused by similar mechanisms.
Graphical Abstract






Similar content being viewed by others
References
Gopalsamy A, Aulabaugh AE, Barakat A, Beaumont KC, Cabral S, Canterbury DP, et al. PF-07059013: a noncovalent modulator of hemoglobin for treatment of sickle cell disease. J Med Chem. 2021;64(1):326–42.
Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–92.
Ahmed MH, Ghatge MS, Safo MK. Hemoglobin: structure, function and allostery. Subcell Biochem. 2020;94:345–82.
Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–31.
Odievre MH, Verger E, Silva-Pinto AC, Elion J. Pathophysiological insights in sickle cell disease. Indian J Med Res. 2011;134(4):532–7.
Nita B. Vasaikar SS, Swati Patil, L.B. Borse, S.L. Borse, S.P. Pawar. 2015. A review on sickle cell anemia.
Meier ER. Treatment options for sickle cell disease. Pediatr Clin North Am. 2018;65(3):427–43.
Blair HA. Voxelotor: first approval. Drugs. 2020;80(2):209–15.
Han J, Saraf SL, Gordeuk VR. Systematic review of voxelotor: a first-in-class sickle hemoglobin polymerization inhibitor for management of sickle cell disease. Pharmacotherapy. 2020;40(6):525–34.
Hoppe C, Neumayr L. Sickle cell disease: monitoring, current treatment, and therapeutics under development. Hematol Oncol Clin North Am. 2019;33(3):355–71.
Study of single and multiple ascending doses of PF-07059013 in healthy adult participants: Clinical Trials.gov; 2022 [Available from: https://clinicaltrials.gov/ct2/show/NCT04323124.
McArthur JG, Svenstrup N, Chen C, Fricot A, Carvalho C, Nguyen J, et al. A novel, highly potent and selective phosphodiesterase-9 inhibitor for the treatment of sickle cell disease. Haematologica. 2020;105(3):623–31.
Knee KM, Jasuja R, Barakat A, Rao D, Wenzel Z, Jasti J, et al. PF-07059013: a non-covalent hemoglobin modulator favorably impacts disease state in a mouse model of sickle cell disease. Am J Hematol. 2021;96(8):E272–5.
Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol. 2016;175(1):141–53.
An G. Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J Clin Pharmacol. 2020;60(2):149–63.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
An G, Liu W, Katz DA, Marek GJ, Awni W, Dutta S. Population pharmacokinetics of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor ABT-384 in healthy volunteers following single and multiple dose regimens. Biopharm Drug Dispos. 2014;35(7):417–29.
Wu N, Katz DA, An G. A target-mediated drug disposition model to explain nonlinear pharmacokinetics of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor SPI-62 in healthy adults. J Clin Pharmacol. 2021;61(11):1442–53.
Wright DH, Stone JA, Crumley TM, Wenning L, Zheng W, Yan K, et al. Pharmacokinetic-pharmacodynamic studies of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor MK-0916 in healthy subjects. Br J Clin Pharmacol. 2013;76(6):917–31.
An G. Small-molecule compounds exhibiting target-mediated drug disposition (TMDD): a minireview. J Clin Pharmacol. 2017;57(2):137–50.
Bach T, Jiang Y, Zhang X, An G. General pharmacokinetic features of small-molecule compounds exhibiting target-mediated drug disposition (TMDD): a simulation-based study. J Clin Pharmacol. 2019;59(3):394–405.
O’Donnell BJ, Guo L, Ghosh S, Shah FA, Strollo PJ Jr, McVerry BJ, et al. Sleep phenotype in the Townes mouse model of sickle cell disease. Sleep Breath. 2019;23(1):333–9.
Sauro HM. Enzyme kinetics for systems biology. 2nd ed. Seattle: Ambrosius Publishing; 2012.
Eaton WA, Henry ER, Hofrichter J, Mozzarelli A. Is cooperative oxygen binding by hemoglobin really understood? Nature Structural Biology. 1999;6(4):351–8.
Leow MK. Configuration of the hemoglobin oxygen dissociation curve demystified: a basic mathematical proof for medical and biological sciences undergraduates. Adv Physiol Educ. 2007;31(2):198–201.
Holt JM, Ackers GK. The Hill coefficient: inadequate resolution of cooperativity in human hemoglobin. Methods Enzymol. 2009;455:193–212.
Adair GS. 1925 The hemoglobin system. V.I The oxygen dissociation curve of hemoglobin. J Biol Chem. 63(2):529-45.
An G, Lee KSS, Yang J, Hammock BD. Target-mediated drug disposition-a class effect of soluble epoxide hydrolase inhibitors. J Clin Pharmacol. 2021;61(4):531–7.
van Waterschoot RAB, Parrott NJ, Olivares-Morales A, Lave T, Rowland M, Smith DA. Impact of target interactions on small-molecule drug disposition: an overlooked area. Nat Rev Drug Discov. 2018;17(4):299.
An G, Katz DA. 2022. Importance of target-mediated drug disposition (TMDD) of small-molecule compounds and its impact on drug development - example of the class effect of HSD-1 inhibitors. J Clin Pharmacol. https://doi.org/10.1002/jcph.2185.
Bohr C, Hasselbalch K, Krogh A. About a new biological relation of high importance that the blood carbonic acid tension exercises on its oxygen binding. Skand Arch Physiol. 1904;16:402–12.
Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(3):599–602.
Hall C, Lueshen E, Mosat A, Linninger AA. Interspecies scaling in pharmacokinetics: a novel whole-body physiologically based modeling framework to discover drug biodistribution mechanisms in vivo. J Pharm Sci. 2012;101(3):1221–41.
van Beekvelt MCP, Colier WNJM, Wevers RA, van Engelen BGM. Performance of near-infrared spectroscopy in measuring local O-2 consumption and blood flow in skeletal muscle. J Appl Physiol. 2001;90(2):511–9.
Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br J Haematol. 1996;93(4):817–21.
Author information
Authors and Affiliations
Contributions
Conception and design of the modeling work: An
Model development: Xu, An
Manuscript preparation: Xu, An
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., An, G. A Pharmacometrics Model to Characterize a New Type of Target-Mediated Drug Disposition (TMDD) – Nonlinear Pharmacokinetics of Small-Molecule PF-07059013 Mediated By Its High-capacity Pharmacological Target Hemoglobin With Positive Cooperative Binding. AAPS J 25, 41 (2023). https://doi.org/10.1208/s12248-023-00808-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00808-3