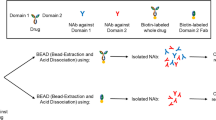
Abstract
Immunogenicity testing to detect and characterize anti-drug antibody (ADA) is required for almost all biotherapeutics. Monoclonal antibody biotherapeutics usually have long half-lives and for high-dose indications such as oncology, high level of drug will be present in the testing samples and interfere with ADA and/or neutralization antibody (NAb) measurement. To overcome this drug interference, acid-dissociation-based sample pre-treatment such as Bead-Extraction and Acid Dissociation (BEAD) has been successfully applied. The main concern for these acid-dissociation-based methods, however, is that harsh acid treatment could denature positive control Abs as well as NAb species in testing samples. In addition, high amount of biotinylated drug is needed in order to have effective competition with high level of drug in the samples, which in turn requires expensive magnetic beads. And the whole process of magnetic beads handling is tedious if doing manually and often causes trouble during assay transfer. Here, we describe a novel method which we named as Precipitation, Acid Dissociation and Biotin-drug as Assay Drug (PABAD). This novel method will need only one step of acid dissociation, with much milder and shorter acid treatment to maximally preserve NAb activity. In addition, only a fraction of biotinylated-drug is needed and there is no need to use additional streptavidin (SA)-plate or SA-magnetic beads for extraction. Compared to a BEAD-based assay, PABAD demonstrates significantly improved recovery of acid-sensitive NAb positive controls (PCs) and similar recovery of acid-resistant NAb PCs.
Graphical Abstract






Similar content being viewed by others
References
Truong TG, Kennedy LB, Patel SP. 25 years of adjuvant therapy in melanoma: a perspective on current approvals and insights into future directions. Curr Oncol Rep. 2022;24(4):533–42.
Cetin B, Wabl CA, Gumusay O. Reshaping treatment paradigms for advanced renal cell cancer patients and improving patient management: optimal management for renal cell cancer patients. Curr Treat Options Oncol. 2022.
Hogner A, Moehler M. Immunotherapy in gastric cancer. Curr Oncol. 2022;29(3):1559–74.
Dodge R, Daus C, Yaskanin D. Challenges in developing antidrug antibody screening assays. Bioanalysis. 2009;1(4):699–704.
FDA. Immunogenicity testing of therapeutic protein products —developing and validating assays for anti-drug antibody detection. 2019.
Casadevall N. Antibodies against rHuEPO: native and recombinant. Nephrol Dial Transplant. 2002;17(Suppl 5):42–7.
Xu W, Cummings J, Sank M, Juhel M, Li X, Gleason C, et al. Development and validation of a functional cell-based neutralizing antibody assay for ipilimumab. Bioanalysis. 2018;10(16):1273–87.
Xu W, Sank M, Cummings J, Carl S, Juhel M, Gleason C, et al. Bead-extraction and heat-dissociation (BEHD): a novel way to overcome drug and matrix interference in immunogenicity testing. J Immunol Methods. 2018;462:34–41.
Lofgren JA, Wala I, Koren E, Swanson SJ, Jing S. Detection of neutralizing anti-therapeutic protein antibodies in serum or plasma samples containing high levels of the therapeutic protein. J Immunol Methods. 2006;308(1–2):101–8.
Wu B, Schnarr M, Devlin JL, Brown S, Yang TY. Approaches to improve drug tolerance and target tolerance in the assessment of neutralizing anti-drug antibodies. Bioanalysis. 2019;11(22):2061–74.
Xiang Y, Parng C, Olson K, Seletskaia E, Gorovits B, Jani D, et al. Neutralizing antibody assay development with high drug and target tolerance to support clinical development of an anti-TFPI therapeutic monoclonal antibody. AAPS J. 2019;21(3):46.
Zoghbi J, Xu Y, Grabert R, Theobald V, Richards S. A breakthrough novel method to resolve the drug and target interference problem in immunogenicity assays. J Immunol Methods. 2015;426:62–9.
Funding
Funding and all the materials that are not commercially available for this research were provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Conception, design, or planning of the study: DW, WX, and RH. Acquisition of the data: DW, JW, and TW.
Corresponding author
Ethics declarations
Conflict of Interest
DW, JW, TW, FF, TS, RH, and WX are current employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wickramarachchi, D., Wagner, J., Woo, T. et al. A Novel Neutralization Antibody Assay Method to Overcome Drug Interference with Better Compatibility with Acid-Sensitive Neutralizing Antibodies. AAPS J 25, 18 (2023). https://doi.org/10.1208/s12248-023-00783-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00783-9